Stereospecific Radical Bromination of β-Aryl Alcohols with Thiourea Additives Through A Serendipitous Discovery of A 1,2-Aryl Migration
- PMID: 39745478
- PMCID: PMC11962344
- DOI: 10.1002/chem.202403831
Stereospecific Radical Bromination of β-Aryl Alcohols with Thiourea Additives Through A Serendipitous Discovery of A 1,2-Aryl Migration
Abstract
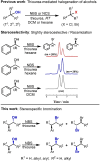
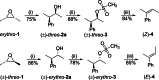
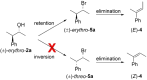
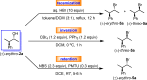
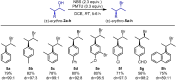
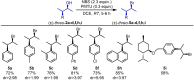
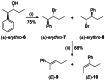
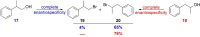
The development of new protocols for stereospecific and stereoselective halogenation transformations by mild reaction conditions is a highly desirable research target for the chemical and pharmaceutical industries. Following the straightforward methodology for directly transforming a wide scope of alcohols to alkyl bromides and chlorides using substoichiometric amounts of thioureas and N-halo succinimides (NXS) as a halogen source in a single step, we noticed that in apolar solvents bromination of chiral secondary alcohols did not produce the expected racemates. In this study, the stereochemical aspects of the bromination reaction were examined. Surprisingly, bromination of (±)-threo- or (±)-erythro-3-phenyl-2-butanols revealed a single diastereomeric brominated product with retention of configuration. The scope of these reactions was expanded on several β-aryl alcohols. During these studies, an unexpected stereospecific 1,2-migration of the phenyl group was shown to take place. The proposed mechanism of the 1,2-phenyl migration involves the formation of a spiro[2,5]octadienyl radical, which is then attacked by a bromide radical at any of the two cyclopropyl positions anti to the phenyl position, leading to products that retain the stereoisomeric configuration of the starting material.
Keywords: Alcohols; Halogenation; Radical aryl migration; Stereoselectivity; Thiourea.
© 2025 The Author(s). Chemistry - A European Journal published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
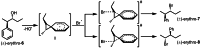
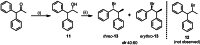
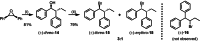
References
-
- Ravindranath B., Perfumer Flavorist 1985, 10, 39–50.
-
- Wagner C., El Omari M., König G. M., J. Nat. Prod. 2009, 72, 540–553. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

