Copper and Silver Trispyrazolylborate-Phosphinoazide Complexes: Synthesis, Characterization, and Nitrene Generation
- PMID: 39745493
- PMCID: PMC11734125
- DOI: 10.1021/acs.inorgchem.4c04397
Copper and Silver Trispyrazolylborate-Phosphinoazide Complexes: Synthesis, Characterization, and Nitrene Generation
Abstract
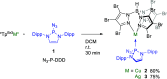
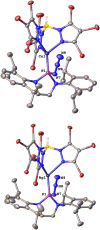
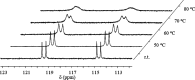
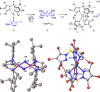
Phosphinoazide complexes of the composition TpBr3M-L (M = Cu, Ag, and L = 2-azido-1,3-bis(2,6-diisopropylphenyl)-2,3-dihydro-1H-1,3,2-diazaphosphole) have been synthesized and structurally characterized. Their thermal decomposition led to cyclodiphosphazenes as a result of the metal-mediated coupling of two nitrene units in a process that takes place in both a stoichiometric and catalytic manner. Experimental data have allowed proposing a mechanistic pathway for this new transformation.
Conflict of interest statement
The authors declare no competing financial interest.
Figures




Similar articles
-
Synthesis and characterization of oxygen-functionalised-NHC silver(I) complexes and NHC transmetallation to nickel(II).Dalton Trans. 2014 Mar 28;43(12):4700-10. doi: 10.1039/c3dt52773e. Dalton Trans. 2014. PMID: 24473851
-
Trispyrazolylborate Ligands Supported on Vinyl Addition Polynorbornenes and Their Copper Derivatives as Recyclable Catalysts.Chemistry. 2019 Jan 7;25(2):556-563. doi: 10.1002/chem.201803852. Epub 2018 Nov 28. Chemistry. 2019. PMID: 30194871
-
Crystal structures and magnetic properties of 2,3,5,6-tetrakis(2-pyridyl)pyrazine (tppz)-containing copper(II) complexes.Inorg Chem. 2003 Dec 29;42(26):8716-27. doi: 10.1021/ic030244v. Inorg Chem. 2003. PMID: 14686849
-
Copper-Catalyzed Sulfimidation in Aqueous Media: a Fast, Chemoselective and Biomolecule-Compatible Reaction.Chemistry. 2024 Mar 7;30(14):e202303939. doi: 10.1002/chem.202303939. Epub 2024 Jan 17. Chemistry. 2024. PMID: 38116945
-
Catalytic synthesis of azoarenes via metal-mediated nitrene coupling.Dalton Trans. 2022 Mar 22;51(12):4577-4589. doi: 10.1039/d2dt00228k. Dalton Trans. 2022. PMID: 35229862 Review.
References
-
- Dequirez G.; Pons V.; Dauban P. Nitrene Chemistry in Organic Synthesis: Still in Its Infancy?. Angew. Chem., Int. Ed. 2012, 51, 7384–7395. 10.1002/anie.201201945. - DOI - PubMed
- Davies H. M. L.; Manning J. R. Catalytic C–H Functionalization by Metal Carbenoid and Nitrenoid Insertion. Nature 2008, 451, 417–424. 10.1038/nature06485. - DOI - PMC - PubMed
-
- Liu Y.; Shing K. P.; Lo V. K.-Y.; Che C.-M. Iron- and Ruthenium-Catalyzed C–N Bond Formation Reactions. Reactive Metal Imido/Nitrene Intermediates. ACS Catal. 2023, 13, 1103–1124. 10.1021/acscatal.2c04830. - DOI
- Reith S.; Demeshko S.; Battistella B.; Reckziegel A.; Schneider C.; Stoy A.; Lichtenberg C.; Meyer F.; Munz D.; Werncke C. G. Between imide, imidyl and nitrene–an imido iron complex in two oxidation states. Chem. Sci. 2022, 13, 7907–7913. 10.1039/D2SC01088G. - DOI - PMC - PubMed
- Grunwald A.; Anjana S. S.; Munz D. Terminal Imido Complexes of the Groups 9–11: Electronic Structure and Developments in the Last Decade. Eur. J. Inorg. Chem. 2021, 2021, 4147–4166. 10.1002/ejic.202100410. - DOI
- Goswami M.; Lyaskovskyy V.; Domingos S. R.; Buma W. J.; Woutersen S.; Troeppner O.; Ivanović-Burmazović I.; Lu H.; Cui X.; Zhang X. P.; Reijerse E. J.; DeBeer S.; van Schooneveld M. M.; Pfaff F. F.; Ray K.; de Bruin B. Characterization of Porphyrin-Co(III)-‘Nitrene Radical’ Species Relevant in Catalytic Nitrene Transfer Reactions. J. Am. Chem. Soc. 2015, 137, 5468–5479. 10.1021/jacs.5b01197. - DOI - PMC - PubMed
- Gouré E.; Avenier F.; Dubourdeaux P.; Sénèque O.; Albriex F.; Lebrun C.; Clémancey M.; Maldivi P.; Latour J.-M. A Diiron(III,IV) Imido Species Very Active in Nitrene-Transfer Reactions. Angew. Chem., Int. Ed. 2014, 53, 1580–1584. 10.1002/anie.201307429. - DOI - PubMed
- Ray K.; Heims F.; Pfaff F. F. Terminal Oxo and Imido Transition-Metal Complexes of Groups 9–11. Eur. J. Inorg. Chem. 2013, 2013, 3784–3807. 10.1002/ejic.201300223. - DOI
- Takaoka A.; Moret M.-E.; Peters J. C. A Ru(I) Metalloradical That Catalyzes Nitrene Coupling to Azoarenes from Arylazides. J. Am. Chem. Soc. 2012, 134, 6695–6706. 10.1021/ja211603f. - DOI - PubMed
- King E. R.; Hennessy E. T.; Betley T. A. Catalytic C-H bond amination from high-spin iron imido complexes. J. Am. Chem. Soc. 2011, 133, 4917–4923. 10.1021/ja110066j. - DOI - PubMed
- Badiei Y. M.; Krishnaswamy A.; Melzer M. M.; Warren T. H. Transient Terminal Cu–Nitrene Intermediates from Discrete Dicopper Nitrenes. J. Am. Chem. Soc. 2006, 128, 15056–15057. 10.1021/ja065299l. - DOI - PubMed
- Shay D. T.; Yap G. P. A.; Zakharov L. N.; Rheingold A. L.; Theopold K. H. Intramolecular C–H Activation by an Open-Shell Cobalt(III) Imido Complex. Angew. Chem., Int. Ed. 2005, 44, 1508–1510. 10.1002/anie.200462529. - DOI - PubMed
-
-
Selected examples of copper-nitrene complexes:
- Carsch K. M.; North S. C.; DiMucci I. M.; Illescu A.; Vojácková P.; Khazanov T.; Zheng S.-L.; Cundari T. R.; Lancaster K. M.; Betley T. A. Electronic Structures and Reactivity Profiles of Aryl Nitrenoid-Bridged Dicopper Complexes. J. Am. Chem. Sci. 2020, 142, 2264–2276. 10.1021/jacs.9b09616. - DOI - PMC - PubMed
- Carsch K. M.; Lukens J. T.; DiMucci I. M.; Iovan D. A.; Zheng S.-L.; Lancaster K. M.; Betley T. A. Electronic Structures and Reactivity Profiles of Aryl Nitrenoid-Bridged Dicopper Complexes. J. Am. Chem. Soc. 2020, 142, 2264–2276. 10.1021/jacs.9b09616. - DOI - PMC - PubMed
- Carsch K. M.; DiMucci I. M.; Iovan D. A.; Li A.; Zheng S.-L.; Titus C. J.; Lee S. J.; Irwin K. D.; Nordlund D.; Lancaster K. M.; Betley T. A. Synthesis of a copper-supported triplet nitrene complex pertinent to copper-catalyzed amination. Science 2019, 365, 1138–1143. 10.1126/science.aax4423. - DOI - PMC - PubMed
- Moegling J.; Hoffmann A.; Thomas F.; Orth N.; Liebhäuser P.; Herber U.; Rampmaier R.; Stanek J.; Fink G.; Ivanovic-Burmazovic I.; Herres-Pawlis S. Designed To React: Terminal Copper Nitrenes and Their Application in Catalytic C–H Aminations. Angew. Chem., Int. Ed. 2018, 57, 9154–9159. 10.1002/anie.201713171. - DOI - PubMed
- Corona T.; Ribas L.; Rovira M.; Farquhar E. R.; Ribas X.; Ray K.; Company A. Characterization and Reactivity Studies of a Terminal Copper–Nitrene Species. Angew. Chem., Int. Ed. 2016, 55, 14005–14008. 10.1002/anie.201607238. - DOI - PMC - PubMed
- Kundu S.; Miceli E.; Farquhar E.; Pfaff F. F.; Kuhlmann U.; Hildebrandt P.; Braun B.; Greco C.; Ray K. Lewis Acid Trapping of an Elusive Copper–Tosylnitrene Intermediate Using Scandium Triflate. J. Am. Chem. Soc. 2012, 134, 14710–14713. 10.1021/ja306674h. - DOI - PMC - PubMed
-
-
- Sicard G.; Baceiredo A.; Bertrand G.; Majoral J.-P. First Evidence for an Intermediate Nitrilo-λ5-phosphane. Angew. Chem., Int. Ed. Engl. 1984, 23, 459–460. 10.1002/anie.198404591. - DOI
-
- Baceiredo A.; Bertrand G.; Majoral J. P.; Sicard G.; Jaud J.; Galy J. Synthesis and structure of the first cyclodiphosphazene. Dimerization of a phosphonitrile. J. Am. Chem. Soc. 1984, 106, 6088–6089. 10.1021/ja00332a061. - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

