Cost-Effectiveness of Breast Cancer Screening Using Digital Mammography in Canada
- PMID: 39745700
- PMCID: PMC11696453
- DOI: 10.1001/jamanetworkopen.2024.52821
Cost-Effectiveness of Breast Cancer Screening Using Digital Mammography in Canada
Abstract
Importance: Evolving breast cancer treatments have led to improved outcomes but carry a substantial financial burden. The association of treatment costs with the cost-effectiveness of screening mammography is unknown.
Objective: To determine the cost-effectiveness of population-based breast cancer screening in the context of current treatment standards.
Design, setting, and participants: In this economic evaluation, the Canadian Partnership Against Cancer/Statistics Canada OncoSim-Breast microsimulation model was used to estimate the impact of various screening schedules in terms of clinical outcomes and treatment costs. Breast cancer treatment costs were derived from activity-based costing published in 2023 specific to a publicly funded health system in Ontario, Canada. A single birth cohort of individuals assigned female at birth in 1975 was modeled until death or age 99 years (whichever came first).
Exposures: Five screening scenarios were modeled: no screening, biennial (ages 50-74 years and 40-74 years), hybrid (biennial ages 40-49 years and annual ages 50-74 years), and annual screening (ages 40-74 years).
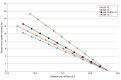
Main outcomes and measures: Incremental cost-effectiveness ratios for deaths averted, life-years (LYs) gained, and incremental cost-utility ratios for quality-adjusted life-years (QALYs) gained were determined for screening scenarios. Sensitivity analyses were conducted by varying screening participation rates and reducing recall rates to 5% and the estimated mortality benefits of screening.
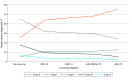
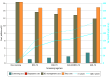
Results: Earlier initiation of breast cancer screening at age 40 years (vs age 50 years) was associated with improved clinical outcomes (deaths averted, LYs saved, and QALYs gained) and reduced health care spending on breast cancer treatment. From a health system perspective, incremental cost-effectiveness ratios for biennial screening at ages 40 to 74 years compared with biennial screening at ages 50 to 74 years were cost saving, with CAD$49 759 saved per death averted, $1558 per LY saved, and $2007 saved per QALY gained. Annual screening at ages 40 to 74 years was cost-effective while achieving the best breast cancer outcomes, with costs of $25 501 per death averted, $1100 per LY saved, and $1447 per QALY gained compared with the current Canadian standard of biennial screening at ages 50 to 74 years.
Conclusions and relevance: In this economic analysis, although screening costs increased according to the number of lifetime screens, they were completely or largely offset by reduced breast cancer therapy costs. Digital mammography was a highly cost-effective tool to reduce breast cancer mortality. These results have important policy implications for all single-payer health systems and call for greater investment in screening programs.
Conflict of interest statement
Figures
References
-
- Canadian Cancer Society . Canadian cancer statistics 2023. 2023. Accessed June 3, 2024. https://cancer.ca/Canadian-Cancer-Statistics-2023-EN
-
- Surveillance Epidemiology and End Results Program; National Cancer Institute. Cancer Stat Facts: female breast cancer. Accessed June 3, 2024. https://seer.cancer.gov/statfacts/html/breast.html
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

