The nanoscale organization of the Nipah virus fusion protein informs new membrane fusion mechanisms
- PMID: 39745781
- PMCID: PMC11695058
- DOI: 10.7554/eLife.97017
The nanoscale organization of the Nipah virus fusion protein informs new membrane fusion mechanisms
Abstract
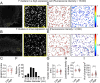
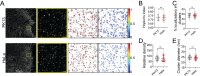
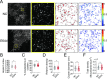
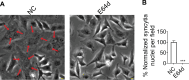
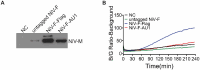
Paramyxovirus membrane fusion requires an attachment protein for receptor binding and a fusion protein for membrane fusion triggering. Nipah virus (NiV) attachment protein (G) binds to ephrinB2 or -B3 receptors, and fusion protein (F) mediates membrane fusion. NiV-F is a class I fusion protein and is activated by endosomal cleavage. The crystal structure of a soluble GCN4-decorated NiV-F shows a hexamer-of-trimer assembly. Here, we used single-molecule localization microscopy to quantify the NiV-F distribution and organization on cell and virus-like particle membranes at a nanometer precision. We found that NiV-F on biological membranes forms distinctive clusters that are independent of endosomal cleavage or expression levels. The sequestration of NiV-F into dense clusters favors membrane fusion triggering. The nano-distribution and organization of NiV-F are susceptible to mutations at the hexamer-of-trimer interface, and the putative oligomerization motif on the transmembrane domain. We also show that NiV-F nanoclusters are maintained by NiV-F-AP-2 interactions and the clathrin coat assembly. We propose that the organization of NiV-F into nanoclusters facilitates membrane fusion triggering by a mixed population of NiV-F molecules with varied degrees of cleavage and opportunities for interacting with the NiV-G/receptor complex. These observations provide insights into the in situ organization and activation mechanisms of the NiV fusion machinery.
Keywords: biochemistry; chemical biology; infectious disease; membrane fusion; microbiology; paramyxovirus; single-molecule localization microscopy; viruses.
© 2024, Wang et al.
Conflict of interest statement
QW, JL, YL, VK, GM, JW, QL No competing interests declared
Figures








Update of
- doi: 10.1101/2024.02.07.579372
- doi: 10.7554/eLife.97017.1
- doi: 10.7554/eLife.97017.2
References
-
- Ankerst M, Breunig MM, Kriegel HP, Sander J. OPTICS: ordering points to identify the clustering structure. SIGMOD Record. 1999;28:49–60. doi: 10.1145/304181.304187. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

