Interrogation of Enantioselectivity in the Photomediated Ring Contractions of Saturated Heterocycles
- PMID: 39746148
- PMCID: PMC12081160
- DOI: 10.1021/jacs.4c13999
Interrogation of Enantioselectivity in the Photomediated Ring Contractions of Saturated Heterocycles
Abstract
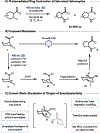
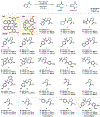
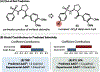
We recently reported a chiral phosphoric acid (CPA) catalyzed enantioselective photomediated ring contraction of piperidines and other saturated heterocycles. By extruding a single heteroatom from a ring, this transformation builds desirable C(sp3)-C(sp3) bonds in the ring contracted products; however, the origins of enantioselectivity remain poorly understood. In this work, enantioselectivity of the ring contraction has been explored across an expanded structurally diverse substrate scope, revealing a wide range of enantioselectivities (0-99%) using two distinct CPA catalysts. Mechanistic investigations support rate-determining excitation that generates short-lived achiral intermediates that are intercepted by the CPA in an enantiodetermining ring closure. The effects of competitive uncatalyzed reactivity and light-driven reversibility of the enantiodetermining ring closure on enantioselectivity have been elucidated. Statistical models were built by regressing the range of enantioselectivities from the substrate scope against key structural features of the products for both CPA catalysts. The resultant models suggested distinct factors that influence the enantioselectivity response for each catalyst and enabled rational modification of a pharmaceutically relevant target molecule to improve enantioselectivity. Finally, density functional theory (DFT)-based transition state analysis identified distinct noncovalent interactions with each catalyst that correlated with the unique selectivity-relevant features uncovered through statistical modeling. Our findings not only offer comprehensive insight into the origins of enantioselectivity in this system but should also aid future development of related photomediated CPA-catalyzed reactions.
Conflict of interest statement
Notes
The authors declare no competing financial interest. R.S. is a paid consultant for MSD.
Figures


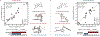


References
-
- Lovering F; Bikker J; Humblet C Escape from Flatland: Increasing Saturation as an Approach to Improving Clinical Success. J. Med. Chem. 2009, 52 (21), 6752–6756. - PubMed
-
- Lovering F Escape from Flatland 2: Complexity and Promiscuity. Med. Chem. Commun. 2013, 4 (3), 515–519.
-
- Campos KR; Coleman PJ; Alvarez JC; Dreher SD; Garbaccio RM; Terrett NK; Tillyer RD; Truppo MD; Parmee ER The Importance of Synthetic Chemistry in the Pharmaceutical Industry. Science 2019, 363 (6424), No. eaat0805. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

