Volumetric Additive Manufacturing for Cell Printing: Bridging Industry Adaptation and Regulatory Frontiers
- PMID: 39746181
- PMCID: PMC11733917
- DOI: 10.1021/acsbiomaterials.4c01837
Volumetric Additive Manufacturing for Cell Printing: Bridging Industry Adaptation and Regulatory Frontiers
Abstract
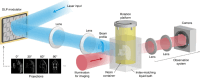
Volumetric additive manufacturing (VAM) is revolutionizing the field of cell printing by enabling the rapid creation of complex three-dimensional cellular structures that mimic natural tissues. This paper explores the advantages and limitations of various VAM techniques, such as holographic lithography, digital light processing, and volumetric projection, while addressing their suitability across diverse industrial applications. Despite the significant potential of VAM, challenges related to regulatory compliance and scalability persist, particularly in the context of bioprinted tissues. In India, the lack of clear regulatory guidelines and intellectual property protections poses additional hurdles for companies seeking to navigate the evolving landscape of bioprinting. This study emphasizes the importance of collaboration among industry stakeholders, regulatory agencies, and academic institutions to establish tailored frameworks that promote innovation while ensuring safety and efficacy. By bridging the gap between technological advancement and regulatory oversight, VAM can unlock new opportunities in regenerative medicine and tissue engineering, transforming patient care and therapeutic outcomes.
Keywords: Volumetric additive manufacturing; additive manufacturing; light-based printing; tomography.
Conflict of interest statement
The authors declare no competing financial interest.
Figures








Similar articles
-
3D Bioprinting for Engineered Tissue Constructs and Patient-Specific Models: Current Progress and Prospects in Clinical Applications.Adv Mater. 2024 Dec;36(49):e2408032. doi: 10.1002/adma.202408032. Epub 2024 Oct 17. Adv Mater. 2024. PMID: 39420757 Review.
-
Volumetric additive manufacturing of pristine silk-based (bio)inks.Nat Commun. 2023 Jan 13;14(1):210. doi: 10.1038/s41467-023-35807-7. Nat Commun. 2023. PMID: 36639727 Free PMC article.
-
Bioprinting and Intellectual Property: Challenges, Opportunities, and the Road Ahead.Bioengineering (Basel). 2025 Jan 15;12(1):76. doi: 10.3390/bioengineering12010076. Bioengineering (Basel). 2025. PMID: 39851350 Free PMC article. Review.
-
3D Bioprinting: from Benches to Translational Applications.Small. 2019 Jun;15(23):e1805510. doi: 10.1002/smll.201805510. Epub 2019 Apr 29. Small. 2019. PMID: 31033203 Free PMC article. Review.
-
Transformative applications of additive manufacturing in biomedical engineering: bioprinting to surgical innovations.J Med Eng Technol. 2024 May;48(4):151-168. doi: 10.1080/03091902.2024.2399017. Epub 2024 Sep 16. J Med Eng Technol. 2024. PMID: 39282861 Review.
Cited by
-
Three-Dimensional Printing and CAD/CAM Milling in Prosthodontics: A Scoping Review of Key Metrics Towards Future Perspectives.J Clin Med. 2025 Jul 8;14(14):4837. doi: 10.3390/jcm14144837. J Clin Med. 2025. PMID: 40725530 Free PMC article. Review.
-
Bioprinting Vascularized Constructs for Clinical Relevance: Engineering Hydrogel Systems for Biological Maturity.Gels. 2025 Aug 12;11(8):636. doi: 10.3390/gels11080636. Gels. 2025. PMID: 40868767 Free PMC article. Review.
-
Transformative bioprinting: 4D printing and its role in the evolution of engineering and personalized medicine.Discov Nano. 2025 Jul 23;20(1):118. doi: 10.1186/s11671-025-04230-w. Discov Nano. 2025. PMID: 40699389 Free PMC article. Review.
-
Next-Generation Wound Healing Materials: Role of Biopolymers and Their Composites.Polymers (Basel). 2025 Aug 19;17(16):2244. doi: 10.3390/polym17162244. Polymers (Basel). 2025. PMID: 40871190 Free PMC article. Review.
References
-
- Bao Y.; Paunović N.; Leroux J. C. Challenges and Opportunities in 3D Printing of Biodegradable Medical Devices by Emerging Photopolymerization Techniques. Adv. Funct. Mater. 2022, 32, 2109864.10.1002/adfm.202109864. - DOI
-
- Bernal P. N.; Bouwmeester M.; Madrid Wolff J.; Falandt M.; Florczak S.; Rodriguez N. G.; Li Y.; Großbacher G.; Samsom R.A.; van Wolferen M.; van der Laan L. J. W.; Delrot P.; Loterie D.; Malda J.; Moser C.; Spee B.; Levato R. Volumetric Bioprinting of Organoids and Optically Tuned Hydrogels to Build Liver-Like Metabolic Biofactories. Adv. Mater. 2022, 34, 2110054.10.1002/adma.202110054. - DOI - PubMed
-
- Joyce M; Hodgkinson T; Lemoine M; Gonzalez-Vazquez A; Kelly D.; O’Brien F. Development of a 3D-Printed Bioabsorbable Composite Scaffold with Mechanical Properties Suitable for Treating Large, Load-Bearing Articular Cartilage Defects. Mater. Sci. Eng., C 2023, 45, 158.10.22203/eCM.v045a11. - DOI - PubMed
-
- Kim D.; Kang D.; Kim D.; Jang J. Volumetric Bioprinting Strategies for Creating Large-Scale Tissues and Organs. MRS Bull. 2023, 48, 657–667. 10.1557/s43577-023-00541-4. - DOI
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Miscellaneous
