Preterm Birth Frequency and Associated Outcomes From the MATISSE (Maternal Immunization Study for Safety and Efficacy) Maternal Trial of the Bivalent Respiratory Syncytial Virus Prefusion F Protein Vaccine
- PMID: 39746206
- PMCID: PMC11731028
- DOI: 10.1097/AOG.0000000000005817
Preterm Birth Frequency and Associated Outcomes From the MATISSE (Maternal Immunization Study for Safety and Efficacy) Maternal Trial of the Bivalent Respiratory Syncytial Virus Prefusion F Protein Vaccine
Abstract
Objective: To describe preterm birth frequency and newborn and infant outcomes overall and among preterm children in the MATISSE (Maternal Immunization Study for Safety and Efficacy) trial of maternal vaccination with bivalent respiratory syncytial virus (RSV) prefusion F protein-based vaccine (RSVpreF) to protect infants against severe RSV-associated illness.
Methods: MATISSE was a global, phase 3, randomized, double-blind trial. Pregnant individuals received single injections of RSVpreF or placebo. Adverse events of special interest, including preterm birth (gestational age less than 37 weeks) and low birth weight (2,500 g or less), were collected through 6 months after delivery (pregnant participants) and from birth through age 12 or 24 months (pediatric participants).
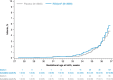
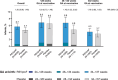
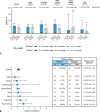
Results: Overall, 7,386 pregnant participants received RSVpreF (n=3,698) or placebo (n=3,688); 7,305 newborns and infants were included in the analysis. Most children in both groups were born full term (more than 93%) with normal birth weight (95% or higher). Newborn and infant outcomes, including rates of low birth weight and neonatal hospitalization, were favorable and comparable between groups. Preterm birth rates were 5.7% in the RSVpreF arm and 4.7% in the placebo arm (relative risk [RR] 1.20, 95% CI, 0.98-1.46); most were late preterm. Newborn and infant outcomes, including rates of low birth weight and neonatal hospitalization, were comparable between groups. Twenty-two newborn or infant deaths occurred during the study (RSVpreF n=8, placebo n=14). When stratified by income region, preterm birth rates in RSVpreF and placebo recipients were both 5.0% in high-income countries. Rates in non-high-income countries were 7.0% and 4.0% in the RSVpreF and placebo groups, respectively, and 8.3% and 4.0% in South Africa (RR 2.06, 95% CI, 1.21-3.51).
Conclusion: In this study of maternal RSVpreF vaccination, no clinically significant increase in adverse events of special interest, including preterm birth, low birth weight, or neonatal hospitalization, was observed among pregnant people in the overall analysis. In subgroup analysis of non-high-income countries, an elevated risk of preterm birth was observed. More research is needed to better ascertain preterm delivery risk factors, particularly aimed at minimizing disparities among geographic regions.
Funding source: This study was sponsored by Pfizer.
Clinical trial registration: ClinicalTrials.gov , NCT04424316.
Copyright © 2024 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures

References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:2047–64. doi: 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- ABRYSVO (RSVpreF): full prescribing information. Pfizer Inc; 2023.
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

