Efficacy, Safety, and Immunogenicity of the MATISSE (Maternal Immunization Study for Safety and Efficacy) Maternal Respiratory Syncytial Virus Prefusion F Protein Vaccine Trial
- PMID: 39746212
- PMCID: PMC11731064
- DOI: 10.1097/AOG.0000000000005816
Efficacy, Safety, and Immunogenicity of the MATISSE (Maternal Immunization Study for Safety and Efficacy) Maternal Respiratory Syncytial Virus Prefusion F Protein Vaccine Trial
Abstract
Objective: To evaluate descriptive efficacy data, exploratory immunogenicity data, and safety follow-up through study completion from the global, phase 3 MATISSE (Maternal Immunization Study for Safety and Efficacy) maternal vaccination trial of bivalent respiratory syncytial virus (RSV) prefusion F protein vaccine (RSVpreF).
Methods: MATISSE was a phase 3, randomized, double-blinded, placebo-controlled trial. Healthy pregnant participants aged 49 years or younger at 24-36 weeks of gestation were randomized (1:1) to receive a single RSVpreF 120 micrograms or placebo dose. Primary efficacy endpoints included newborn and infant severe RSV-associated medically attended lower respiratory tract illness within 180 days after birth. The RSV-A and RSV-B serum neutralizing antibody titers were determined in a subset of pregnant participants and their newborns.
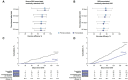
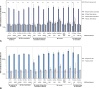
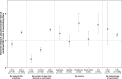
Results: In this final analysis, 7,420 pregnant participants were randomized, and 7,307 children were born (RSVpreF n=3,660, placebo n=3,647). Vaccine efficacy , defined as protection against newborn and infant severe RSV-associated medically attended lower respiratory tract illness, was 82.4% (95% CI, 57.5-93.9) and 70.0% (95% CI, 50.6-82.5) within 90 and 180 days of birth, respectively. The RSVpreF induced robust immune responses in pregnant participants and resulted in highly efficient transfer of maternal antibodies to their newborns across subgroups (by gestational age at delivery and at vaccination, number of days from vaccination to delivery, country, maternal age). Final RSVpreF safety results in pregnant and newborn and infant participants were consistent with the primary analysis with no new safety concerns identified.
Conclusion: This final analysis of MATISSE trial data confirms the primary analysis conclusions: Maternal vaccination with RSVpreF has a favorable safety profile in both pregnant and newborn and infant participants and demonstrates efficacy against RSV-associated lower respiratory tract illness in infants through age 6 months. The RSVpreF induces robust immune responses in pregnant individuals, with corresponding high RSV-neutralizing titers in their newborns.
Clinical trial registration: ClinicalTrials.gov , NCT04424316.
Copyright © 2025 The Author(s). Published by Wolters Kluwer Health, Inc.
Figures


References
-
- Li Y, Wang X, Blau DM, Caballero MT, Feikin DR, Gill CJ, et al. Global, regional, and national disease burden estimates of acute lower respiratory infections due to respiratory syncytial virus in children younger than 5 years in 2019: a systematic analysis. Lancet 2022;399:2047–64. doi: 10.1016/S0140-6736(22)00478-0 - DOI - PMC - PubMed
-
- Fitzpatrick MC, Laufer RS, Baral R, Driscoll AJ, Feikin DR, Fleming JA, et al. Report of the WHO technical consultation on the evaluation of respiratory syncytial virus prevention cost effectiveness in low- and middle-income countries, April 7-8, 2022. Vaccine 2023;41:7047–59. doi: 10.1016/j.vaccine.2023.09.040 - DOI - PMC - PubMed
-
- U.S. Centers for Disease Control and Prevention. Respiratory syncytial virus infection (RSV). Clinical overview of RSV. Accessed December 13, 2024. https://www.cdc.gov/rsv/hcp/clinical-overview/?CDC_AAref_Val=https://www...
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

