Real-time CBCT imaging and motion tracking via a single arbitrarily-angled x-ray projection by a joint dynamic reconstruction and motion estimation (DREME) framework
- PMID: 39746309
- PMCID: PMC11747166
- DOI: 10.1088/1361-6560/ada519
Real-time CBCT imaging and motion tracking via a single arbitrarily-angled x-ray projection by a joint dynamic reconstruction and motion estimation (DREME) framework
Abstract
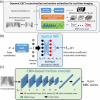
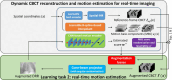
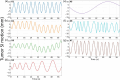
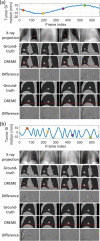
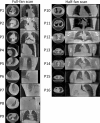
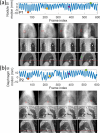
Objective.Real-time cone-beam computed tomography (CBCT) provides instantaneous visualization of patient anatomy for image guidance, motion tracking, and online treatment adaptation in radiotherapy. While many real-time imaging and motion tracking methods leveraged patient-specific prior information to alleviate under-sampling challenges and meet the temporal constraint (<500 ms), the prior information can be outdated and introduce biases, thus compromising the imaging and motion tracking accuracy. To address this challenge, we developed a framework
Keywords: deep learning; dynamic CBCT reconstruction; motion estimation; motion model; real-time imaging; x-ray.
Creative Commons Attribution license.
Figures






Update of
-
Real-time CBCT Imaging and Motion Tracking via a Single Arbitrarily-angled X-ray Projection by a Joint Dynamic Reconstruction and Motion Estimation (DREME) Framework.ArXiv [Preprint]. 2024 Sep 25:arXiv:2409.04614v2. ArXiv. 2024. Update in: Phys Med Biol. 2025 Jan 21;70(2). doi: 10.1088/1361-6560/ada519. PMID: 39398221 Free PMC article. Updated. Preprint.
Similar articles
-
A dynamic reconstruction and motion estimation framework for cardiorespiratory motion-resolved real-time volumetric MR imaging (DREME-MR).ArXiv [Preprint]. 2025 Jul 2:arXiv:2503.21014v2. ArXiv. 2025. Update in: Phys Med Biol. 2025 Aug 08. doi: 10.1088/1361-6560/adf9b9. PMID: 40196152 Free PMC article. Updated. Preprint.
-
Time-resolved dynamic CBCT reconstruction using prior-model-free spatiotemporal Gaussian representation (PMF-STGR).Phys Med Biol. 2025 Aug 12;70(16):165011. doi: 10.1088/1361-6560/adf487. Phys Med Biol. 2025. PMID: 40712635 Free PMC article.
-
A dynamic reconstruction and motion estimation framework for cardiorespiratory motion-resolved real-time volumetric MR imaging (DREME-MR).Phys Med Biol. 2025 Aug 8;70(17):175013. doi: 10.1088/1361-6560/adf9b9. Online ahead of print. Phys Med Biol. 2025. PMID: 40780250 Free PMC article.
-
Real-time CBCT Imaging and Motion Tracking via a Single Arbitrarily-angled X-ray Projection by a Joint Dynamic Reconstruction and Motion Estimation (DREME) Framework.ArXiv [Preprint]. 2024 Sep 25:arXiv:2409.04614v2. ArXiv. 2024. Update in: Phys Med Biol. 2025 Jan 21;70(2). doi: 10.1088/1361-6560/ada519. PMID: 39398221 Free PMC article. Updated. Preprint.
-
Dynamic cone-beam CT reconstruction using spatial and temporal implicit neural representation learning (STINR).Phys Med Biol. 2023 Feb 6;68(4):045005. doi: 10.1088/1361-6560/acb30d. Phys Med Biol. 2023. PMID: 36638543 Free PMC article.
Cited by
-
A dynamic reconstruction and motion estimation framework for cardiorespiratory motion-resolved real-time volumetric MR imaging (DREME-MR).ArXiv [Preprint]. 2025 Jul 2:arXiv:2503.21014v2. ArXiv. 2025. Update in: Phys Med Biol. 2025 Aug 08. doi: 10.1088/1361-6560/adf9b9. PMID: 40196152 Free PMC article. Updated. Preprint.
-
An open-source deep learning framework for respiratory motion monitoring and volumetric imaging during radiation therapy.Med Phys. 2025 Jul;52(7):e18015. doi: 10.1002/mp.18015. Med Phys. 2025. PMID: 40665474 Free PMC article.
-
Time-resolved dynamic CBCT reconstruction using prior-model-free spatiotemporal Gaussian representation (PMF-STGR).Phys Med Biol. 2025 Aug 12;70(16):165011. doi: 10.1088/1361-6560/adf487. Phys Med Biol. 2025. PMID: 40712635 Free PMC article.
-
A dynamic reconstruction and motion estimation framework for cardiorespiratory motion-resolved real-time volumetric MR imaging (DREME-MR).Phys Med Biol. 2025 Aug 8;70(17):175013. doi: 10.1088/1361-6560/adf9b9. Online ahead of print. Phys Med Biol. 2025. PMID: 40780250 Free PMC article.
References
-
- Feldkamp L A, Davis L C, Kress J W. Practical cone-beam algorithm. J. Opt. Soc. Am. A. 1984;1:612–9. doi: 10.1364/JOSAA.1.000612. - DOI
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
