Eye Movements during Measurements of Visual Vertical in the Poststroke Subacute Phase
- PMID: 39746806
- PMCID: PMC11747974
- DOI: 10.1523/ENEURO.0279-24.2024
Eye Movements during Measurements of Visual Vertical in the Poststroke Subacute Phase
Abstract
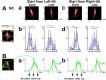
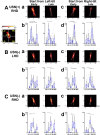
The subjective visual vertical (VV), the visually estimated direction of gravity, is essential for assessing vestibular function and visuospatial cognition. In this study, we aimed to investigate the mechanisms underlying altered VV perception in stroke participants with unilateral spatial neglect (USN), specifically by examining their eye movement patterns during VV judgment tasks. Participants with USN demonstrated limited eye movement scanning along a rotating bar, often fixating on prominent ends, such as the top or bottom. This suggests a reflexive response to visually salient areas, potentially interfering with accurate VV perception. In contrast, participants without USN showed broader scanning around the center of the bar. Notably, participants with USN without frontal lobe lesions occasionally exhibited extended scanning that included the bar's center, which was associated with accurate VV judgments. These findings suggest that (1) a tendency to fixate on peripheral, prominent areas and (2) frontal lobe involvement in disengaging and redirecting spatial attention may influence VV perception in USN. Based on these results, targeted rehabilitation strategies that encourage individuals with USN to extend their visual scanning beyond prominent endpoints and include central areas could improve VV accuracy. This study highlights the specific eye movement behaviors contributing to VV misperception, emphasizing the importance of training that broadens scanning to improve VV perception effectively.
Keywords: disengagement; eye movements; stroke; subjective visual vertical; unilateral spatial neglect.
Copyright © 2025 Arima et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures





References
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
