Optical Assay of the Functional Impact of Cuprizone-Induced Demyelination and Remyelination on Interhemispheric Neural Communication in the Anterior Cingulate Cortex via the Corpus Callosum
- PMID: 39746809
- PMCID: PMC11703560
- DOI: 10.1523/ENEURO.0511-24.2024
Optical Assay of the Functional Impact of Cuprizone-Induced Demyelination and Remyelination on Interhemispheric Neural Communication in the Anterior Cingulate Cortex via the Corpus Callosum
Abstract
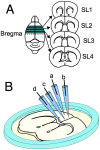
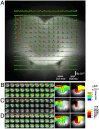
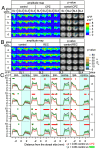
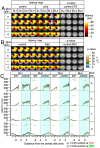
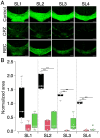
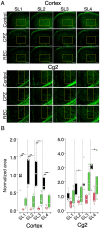
Cuprizone (CPZ) is a widely used toxin that induces demyelinating diseases in animal models, producing multiple sclerosis (MS)-like pathology in rodents. CPZ is one of the few toxins that triggers demyelination and subsequent remyelination following the cessation of its application. This study examines the functional consequences of CPZ-induced demyelination and the subsequent recovery of neural communication within the anterior cingulate cortex (ACC), with a particular focus on interhemispheric connectivity via the corpus callosum (CC). By employing wide-field, high-speed, voltage-sensitive dye imaging, we were able to provide real-time mapping of neural activity in the ACC of CPZ-fed mice. Although we could not record physiological signals from the CC, the results demonstrated a notable impairment in interhemispheric connections within the ACC via the CC, with the most pronounced loss observed in a specific coronal slice among a series of slices examined. Notably, the latency of neural signal propagation remained largely unaltered despite connectivity loss, indicating that demyelination affects the extent, rather than the temporal dynamics, of neural communication. It is noteworthy that while functional connectivity appeared to recover fully after the cessation of CPZ, histological analysis revealed only partial recovery of myelination, indicating a discrepancy between functional and structural recovery. These findings enhance our understanding of how demyelination affects the ACC's role in orchestrating neural activity, particularly in light of the slice-specific nature of interhemispheric communication impairments. These findings offer new insights into MS pathology, particularly regarding the role of the CC in interhemispheric communication and potential therapeutic strategies.
Keywords: anterior cingulate cortex; corpus callosum; cuprizone; medial prefrontal cortex; multiple sclerosis; voltage-sensitive dye.
Copyright © 2025 Tsukuda et al.
Conflict of interest statement
The authors declare no competing financial interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
