Remodelling of the immune landscape by IFNγ counteracts IFNγ-dependent tumour escape in mouse tumour models
- PMID: 39746898
- PMCID: PMC11696141
- DOI: 10.1038/s41467-024-54791-0
Remodelling of the immune landscape by IFNγ counteracts IFNγ-dependent tumour escape in mouse tumour models
Abstract
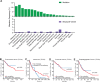
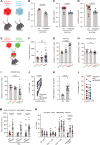
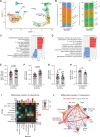
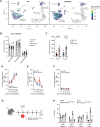
Loss of IFNγ-sensitivity by tumours is thought to be a mechanism enabling evasion, but recent studies suggest that IFNγ-resistant tumours can be sensitised for immunotherapy, yet the underlying mechanism remains unclear. Here, we show that IFNγ receptor-deficient B16-F10 mouse melanoma tumours are controlled as efficiently as WT tumours despite their lower MHC class I expression. Mechanistically, IFNγ receptor deletion in B16-F10 tumours increases IFNγ availability, triggering a remodelling of the immune landscape characterised by inflammatory monocyte infiltration and the generation of 'mono-macs'. This altered myeloid compartment synergises with an increase in antigen-specific CD8+ T cells to promote anti-tumour immunity against IFNγ receptor-deficient tumours, with such an immune crosstalk observed around blood vessels. Importantly, analysis of transcriptomic datasets suggests that similar immune remodelling occurs in human tumours carrying mutations in the IFNγ pathway. Our work thus serves mechanistic insight for the crosstalk between tumour IFNγ resistance and anti-tumour immunity, and implicates this regulation for future cancer therapy.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

