Carbene-catalyzed chirality-controlled site-selective acylation of saccharides
- PMID: 39746955
- PMCID: PMC11697312
- DOI: 10.1038/s41467-024-55282-y
Carbene-catalyzed chirality-controlled site-selective acylation of saccharides
Abstract
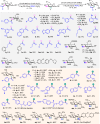
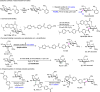
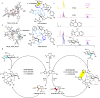
Acylation stands as a fundamental process in both biological pathways and synthetic chemical reactions, with acylated saccharides and their derivatives holding diverse applications ranging from bioactive agents to synthetic building blocks. A longstanding objective in organic synthesis has been the site-selective acylation of saccharides without extensive pre-protection of alcohol units. In this study, we demonstrate that by simply altering the chirality of N-heterocyclic carbene (NHC) organic catalysts, the site-selectivity of saccharide acylation reactions can be effectively modulated. Our investigation reveals that this intriguing selectivity shift stems from a combination of factors, including chirality match/mismatch and inter- / intramolecular hydrogen bonding between the NHC catalyst and saccharide substrates. These findings provide valuable insights into catalyst design and reaction engineering, highlighting potential applications in glycoside analysis, such as fluorescent labelling, α/β identification, orthogonal reactions, and selective late-stage modifications.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Fang, L. L., Xiao, L., Jun, Y. W., Onishi, Y. & Kool, E. T. Reversible 2′-OH acylation enhances RNA stability. Nat. Chem. 15, 1296–1305 (2023). - PubMed
-
- Zhou, Y. et al. Nε-Fatty acylation of Rho GTPases by a MARTX toxin effector. Science358, 528–530 (2017). - PubMed
-
- Xu, Y. et al. A light-driven enzymatic enantioselective radical acylation. Nature625, 74–78 (2024). - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

