A proof-of-concept study in small and large animal models for coupling liver normothermic machine perfusion with mesenchymal stromal cell bioreactors
- PMID: 39746966
- PMCID: PMC11697227
- DOI: 10.1038/s41467-024-55217-7
A proof-of-concept study in small and large animal models for coupling liver normothermic machine perfusion with mesenchymal stromal cell bioreactors
Abstract
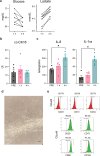
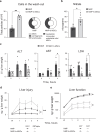
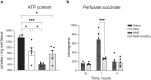
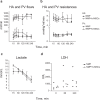
To fully harness mesenchymal-stromal-cells (MSCs)' benefits during Normothermic Machine Perfusion (NMP), we developed an advanced NMP platform coupled with a MSC-bioreactor and investigated its bio-molecular effects and clinical feasibility using rat and porcine models. The study involved three work packages: 1) Development (n = 5): MSC-bioreactors were subjected to 4 h-liverless perfusion; 2) Rat model (n = 10): livers were perfused for 4 h on the MSC-bioreactor-circuit or with the standard platform; 3) Porcine model (n = 6): livers were perfused using a clinical device integrated with a MSC-bioreactor or in its standard setup. MSCs showed intact stem-core properties after liverless-NMP. Liver NMP induced specific, liver-tailored, changes in MSCs' secretome. Rat livers exposed to bioreactor-based perfusion produced more bile, released less damage and pro-inflammatory biomarkers, and showed improved mithocondrial function than those subjected to standard NMP. MSC-bioreactor integration into a clinical device resulted in no machine failure and perfusion-related injury. This proof-of-concept study presents a novel MSC-based liver NMP platform that could reduce the deleterious effects of ischemia/reperfusion before transplantation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures



References
-
- Parente, A. et al. Machine perfusion techniques for liver transplantation - A meta-analysis of the first seven randomized controlled trials. J. Hepatol. 10.1016/J.JHEP.2023.05.027 (2023). - PubMed
-
- Liew, B. et al. Liver transplant outcomes after ex vivo machine perfusion: a meta-analysis. Br. J. Surg.108, 1409–1416 (2021). - PubMed
-
- Lonati, C. et al. Influence of ex vivo perfusion on the biomolecular profile of rat lungs. FASEB J. fj.201701255R (2018). - PubMed
-
- Jassem, W. et al. Normothermic machine perfusion (nmp) inhibits proinflammatory responses in the liver and promotes regeneration. Hepatology70, 682–695 (2019). - PubMed
-
- Gatti, S. et al. Reduced expression of the melanocortin-1 receptor in human liver during brain death. Neuroimmunomodulation13, 51–55 (2006). - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

