G-quadruplex stabilization provokes DNA breaks in human PKD1, revealing a second hit mechanism for ADPKD
- PMID: 39747084
- PMCID: PMC11696556
- DOI: 10.1038/s41467-024-55684-y
G-quadruplex stabilization provokes DNA breaks in human PKD1, revealing a second hit mechanism for ADPKD
Abstract
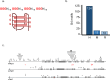
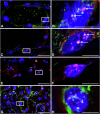
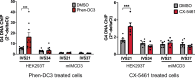
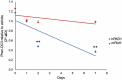
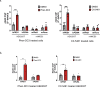
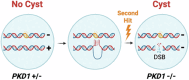
The "secondhit" pathway is responsible for biallelic inactivation of many tumor suppressors, where a pathogenic germline allele is joined by somatic mutation of the remaining functional allele. The mechanisms are unresolved, but the human PKD1 tumor suppressor is a good experimental model for identifying the molecular determinants. Inactivation of PKD1 results in autosomal dominant polycystic kidney disease, a very common disorder characterized by the accumulation of fluid-filled cysts and end-stage renal disease. Since human PKD1 follows second hit and mouse Pkd1 heterozygotes do not, we reasoned that there is likely a molecular difference that explains the elevated mutagenesis of the human gene. Here we demonstrate that guanine quadruplex DNA structures are abundant throughout human, but not mouse, PKD1 where they activate the DNA damage response. Our results suggest that guanine quadruplex DNAs provoke DNA breaks in PKD1, providing a potential mechanism for cystogenesis in autosomal dominant polycystic kidney disease specifically and for the inactivation of guanine quadruplex-rich tumor suppressors generally.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: A pending U.S. Provisional Patent Application No. 63/693,922 (inventor E.L.) directed to G4 quadruplex structures in the PKD1 gene has been filed on behalf of the applicant, Western Michigan University Homer Stryker M.D. School of Medicine. The authors declare no other competing interests.
Figures

References
-
- Watnick, T. J. et al. Somatic mutation in individual liver cysts supports a two-hit model of cystogenesis in autosomal dominant polycystic kidney disease one possible explanation for the focal nature of renal cyst formation. Mol. Cell2, 247–251 (1998). - PubMed
-
- Koptides, M. et al. Loss of heterozygosity in polycystic kidney disease with a missense mutation in the repeated region of PKD1. Hum. Genet.103, 709–717 (1998). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

