Engineering 2D spin networks by on-surface encapsulation of azafullerene radicals in nanotemplates
- PMID: 39747106
- PMCID: PMC11695733
- DOI: 10.1038/s41467-024-55521-2
Engineering 2D spin networks by on-surface encapsulation of azafullerene radicals in nanotemplates
Abstract
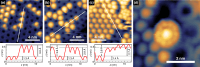
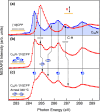
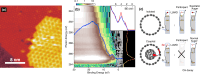
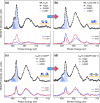
We present an efficient strategy for on-surface engineering of organic metal-free supramolecular complexes with long-term spin protection. By vacuum deposition of azafullerene (C59N•) monomers on a pre-deposited template layer of [10]cycloparaphenylene ([10]CPP) nanohoops on Au(111) surface we exploit the molecular shape matching between the C59N• and [10]CPP for the azafullerene encapsulation with nanohoops in a guest-host complexation geometry. C59N•⊂[10]CPP supramolecular complexes self-assemble into an extended two-dimensional hexagonal lattice yielding a high density network of stable spin-1/2 radicals. We find compelling evidence for electronic coupling between the guest C59N• and the host [10]CPP in supramolecular species. At the same time, [10]CPP effectively protects the radical state of encapsulated azafullerenes against dimerization and inhibits C59N• coupling to the Au substrate. Azafullerene encapsulation by nanohoops represents a viable realization of molecular spin protection while simultaneously demonstrating exceptional self-assembling properties by which large-scale 2D architectures of molecular spins can be realized.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Gaita-Ariño, A., Luis, F., Hill, S. & Coronado, E. Molecular spins for quantum computation. Nat. Chem.11, 301–309 (2019). - PubMed
-
- Köbke, A. et al. Reversible coordination-induced spin-state switching in complexes on metal surfaces. Nat. Nanotechnol.15, 18–21 (2020). - PubMed
-
- Morton, J. J. L. et al. High fidelity single qubit operations using pulsed electron paramagnetic resonance. Phys. Rev. Lett.95, 200501 (2005). - PubMed
-
- Morton, J. J. L. et al. Electron spin relaxation of N@C60 in CS2. J. Chem. Phys. 124, 14508 (2006). - PubMed
-
- Morton, J. J. L. et al. Solid-state quantum memory using the 31P nuclear spin. Nature455, 1085–1088 (2008).
Grants and funding
LinkOut - more resources
Full Text Sources

