Downregulation of Gldc attenuates myocardial ischemia reperfusion injury in vitro by modulating Akt and NF-κB signalings
- PMID: 39747134
- PMCID: PMC11696683
- DOI: 10.1038/s41598-024-79445-5
Downregulation of Gldc attenuates myocardial ischemia reperfusion injury in vitro by modulating Akt and NF-κB signalings
Abstract
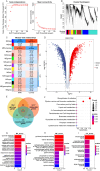
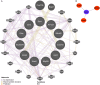
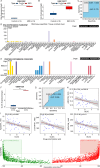
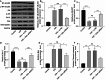
Myocardial ischemia/reperfusion injury (MIRI) is a serious clinical complication that is caused by reperfusion therapy following myocardial infarction (MI). Mitochondria-related genes (Mito-RGs) play important roles in multiple diseases. However, the role of mitochondria-related genes in MIRI remains largely unknown. The GSE67308 dataset from the GEO database was utilized to identify MIRI-related gene modules through WGCNA. Meanwhile, differential expression analysis was conducted to identify differentially expressed genes (DEGs) in the GSE61592 dataset. Next, candidate Mito-RGs related to MIRI were screened by Venn analysis. Thereafter, a myocardial hypoxia/reperfusion (H/R) H9C2 cell model and a mouse ischemia/reperfusion (I/R) model were established to verify the expression level of glycine decarboxylase (Gldc) in MIRI in vitro and in vivo. Based on data from the GEO database, Gldc levels were notably upregulated in murine MIRI samples, compared to the control group. RT-qPCR and western blot confirmed that Gldc levels were obviously elevated in the heart of I/R mice and H/R-exposed cardiomyocytes. Moreover, the deficiency of Gldc notably increased the viability and reduced the apoptosis and inflammatory responses in H9C2 cells exposed to H/R. Meanwhile, Gldc downregulation significantly reduced p-NF-κB p65, Bax and cleaved caspase 3 levels and elevated p-Akt and Bcl-2 levels in H9C2 cells exposed to H/R. The ROC curve analysis further demonstrated that Gldc gene exhibited good diagnostic value for MIRI. Collectively, Gldc deficiency could attenuate H/R injury in cardiomyocytes in vitro through activating Akt and inactivating NF-κB signalings. These data suggested that GLDC may serve as both a diagnostic and therapeutic target for MIRI.
Keywords: Glycine decarboxylase; Mitochondria-related gene; Myocardial infarction; Myocardial ischemia; Myocardial ischemia/Reperfusion injury.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All the animal experiments were complied with the guidelines of the Tianjin Medical Experimental Animal Care, and animal protocols were approved by the Institutional Animal Care and Use Committee of Yi Shengyuan Gene Technology (Tianjin) Co., Ltd. (protocol number YSY-DWLL-2023254).
Figures




References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

