Synergistic design of CuO/CoFe₂O₄/MWCNTs ternary nanocomposite for enhanced photocatalytic degradation of tetracycline under visible light
- PMID: 39747156
- PMCID: PMC11696163
- DOI: 10.1038/s41598-024-82926-2
Synergistic design of CuO/CoFe₂O₄/MWCNTs ternary nanocomposite for enhanced photocatalytic degradation of tetracycline under visible light
Abstract
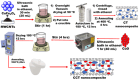
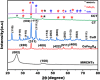
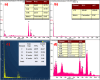
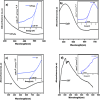
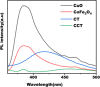
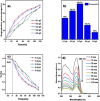
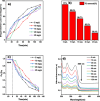
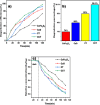
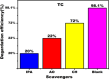
This study involves a novel CuO/CoFe₂O₄/MWCNTs (CCT) nanocomposite, developed by integrating cobalt ferrite (CoFe₂O₄) and copper oxide (CuO) nanoparticles onto multi-walled carbon nanotubes (MWCNTs), for the degradation of tetracycline (TC) under visible light. The photocatalyst was extensively characterized using XRD, HR-SEM, EDX, HR-TEM, UV-Vis, BET, and PL analysis. The synthesized CoFe₂O₄ and CuO nanoparticles exhibited crystallite sizes of 46.8 nm and 37.5 nm, respectively, while the CCT nanocomposite had a crystallite size of 53 nm. Microscopy confirmed a particle size of 49.2 nm for the nanocomposite, with MWCNTs measuring 15.65 nm in diameter. The band gap energy of the CCT nanocomposite was 1.6 eV, which contributed to its enhanced photocatalytic activity, as evidenced by the lower emission intensity in PL analysis. BET analysis revealed a pore volume of 0.37 cc/g and a surface area of 82.3 m²/g. Photocatalytic performance was tested across various conditions, with adjustments to nanocomposite dosages (0.1-0.5 g/L), TC concentrations (5-25 mg/L), and pH levels (2-10). Under optimized conditions (0.3 g/L CCT, 5 mg/L TC, pH 10, 120 min of visible light exposure), the CCT achieved 98.1% degradation of TC. The optimized parameters were subsequently used to assess TC degradation with individual photocatalysts: CoFe₂O₄, CuO, CT, and CCT. The enhanced photocatalytic efficiency observed can be largely attributed to the improved charge transfer dynamics and effective electron-hole separation facilitated by MWCNT doping. The reaction followed a pseudo-first-order kinetic model, with hydroxyl radicals (OH•) identified as the key species in the degradation process. Moreover, the catalyst exhibited 96% retention of its photocatalytic efficiency after five consecutive cycles, demonstrating exceptional stability and reusability. These results emphasize the CCT composite's potential as a highly efficient and sustainable photocatalyst for the remediation of pharmaceutical pollutants in aquatic systems.
Keywords: CuO/CoFe2O4/MWCNTs; Degradation kinetics; Photocatalysis; Tetracycline; Visible light.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures







References
-
- Yadav, S. et al. Highly Efficient Visible-Light-Driven Photocatalysis of Rose Bengal Dye and Hydrogen Production Using Ag@Cu/TiO2 Ternary Nanocomposites. Chem. (Easton). 6, 489–505 (2024).
-
- Vidhya, N. et al. Hydrothermally Synthesized TiO2-Doped MnO2 Nanocomposites for Electrochemical and Photocatalytic Activity. Nano245014310.1142/S1793292024501431 (2024).
-
- Kumar, R., Ansari, S. A., Barakat, M. A., Aljaafari, A. & Cho, M. H. A polyaniline@MoS2-based organic–inorganic nanohybrid for the removal of Congo red: adsorption kinetic, thermodynamic and isotherm studies. New J. Chem.42, 18802–18809 (2018). - DOI
LinkOut - more resources
Full Text Sources
Miscellaneous

