Method for long-term room temperature storage of mouse freeze-dried sperm
- PMID: 39747170
- PMCID: PMC11695616
- DOI: 10.1038/s41598-024-83350-2
Method for long-term room temperature storage of mouse freeze-dried sperm
Abstract
Permanent preservation of genetic resources may be indispensable for the future of humanity. This requires liquid nitrogen, as is the case for preserving animal sperm. However, this technique is expensive and poses a risk of irrecoverable sample loss on non-replenishment of liquid nitrogen in case of natural disasters. In this study, we demonstrate that lyophilization may be used as a reliable method for long-term preservation of mouse sperm at room temperature. Sperm from four mouse strains were freeze-dried and stored in a non-temperature controlled room for 5-6 years. Although the ability of the stored sperm to activate oocytes had diminished slightly, healthy offspring were obtained by artificially activating the oocytes after sperm injection. Moreover, the birth rate did not decrease even after ≤ 6 years of storage. Furthermore, owing to its low cost, safety, and ease of storage at any location, we believe that this method could be a major mode of preserving mammalian genetic resources in the future.
Keywords: Freeze-drying; Genetic resources; Oocyte activation; Room temperature; Sperm preservation.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

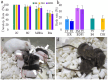
Similar articles
-
Production of Healthy Mice from Freeze-Dried Spermatozoa Preserved at Room Temperature.Methods Mol Biol. 2025;2897:563-575. doi: 10.1007/978-1-0716-4406-5_37. Methods Mol Biol. 2025. PMID: 40202660
-
Long-term preservation of mouse spermatozoa after freeze-drying and freezing without cryoprotection.Biol Reprod. 2003 Dec;69(6):2100-8. doi: 10.1095/biolreprod.103.020529. Epub 2003 Aug 20. Biol Reprod. 2003. PMID: 12930716
-
Assessment of three generations of mice derived by ICSI using freeze-dried sperm.Zygote. 2009 Aug;17(3):239-51. doi: 10.1017/S0967199409005292. Epub 2009 May 6. Zygote. 2009. PMID: 19416557 Free PMC article.
-
Challenging endeavour for preservation of freeze-dried mammalian spermatozoa.J Reprod Dev. 2011 Oct;57(5):557-63. doi: 10.1262/jrd.11-061o. J Reprod Dev. 2011. PMID: 22052044 Review.
-
Freeze-dried spermatozoa: A future tool?Reprod Domest Anim. 2017 Apr;52 Suppl 2:248-254. doi: 10.1111/rda.12838. Epub 2016 Oct 18. Reprod Domest Anim. 2017. PMID: 27757990 Review.
Cited by
-
Monosodium glutamate enhances freeze-dried mouse sperm quality during room-temperature storage.Sci Rep. 2025 Aug 23;15(1):30994. doi: 10.1038/s41598-025-16635-9. Sci Rep. 2025. PMID: 40849549 Free PMC article.
-
Can Humanity Thrive Beyond the Galaxy?J Reprod Dev. 2025 Feb 5;71(1):10-16. doi: 10.1262/jrd.2024-099. Epub 2024 Dec 29. J Reprod Dev. 2025. PMID: 39756865 Free PMC article. Review.
References
-
- Comizzoli, P., Amelkina, O. & Lee, P. C. Damages and stress responses in sperm cells and other germplasms during dehydration and storage at nonfreezing temperatures for fertility preservation. Mol. Reprod. Dev.89, 565–578. 10.1002/mrd.23651 (2022). - PubMed
-
- Loi, P., Iuso, D., Czernik, M., Zacchini, F. & Ptak, G. Towards storage of cells and gametes in dry form. Trends Biotechnol.31, 688–695. 10.1016/j.tibtech.2013.09.004 (2013). - PubMed
-
- Saragusty, J. et al. Dry biobanking as a conservation tool in the Anthropocene. Theriogenology150, 130–138. 10.1016/j.theriogenology.2020.01.022 (2020). - PubMed
-
- Nollet, K. E., Komazawa, T. & Ohto, H. Transfusion under triple threat: Lessons from Japan’s 2011 earthquake, tsunami, and nuclear crisis. Transfus. Apher Sci.55, 177–183. 10.1016/j.transci.2016.09.009 (2016). - PubMed
-
- Tateno, H., Wakayama, T., Ward, W. S. & Yanagimachi, R. Can alcohol retain the reproductive and genetic potential of sperm nuclei? Chromosome analysis of mouse spermatozoa stored in alcohol. Zygote6, 233–238. 10.1017/s0967199498000173 (1998). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

