Targeting pleuro-alveolar junctions reverses lung fibrosis in mice
- PMID: 39747171
- PMCID: PMC11696612
- DOI: 10.1038/s41467-024-55596-x
Targeting pleuro-alveolar junctions reverses lung fibrosis in mice
Erratum in
-
Author Correction: Targeting pleuro-alveolar junctions reverses lung fibrosis in mice.Nat Commun. 2025 Feb 24;16(1):1911. doi: 10.1038/s41467-025-57261-3. Nat Commun. 2025. PMID: 39994215 Free PMC article. No abstract available.
Abstract
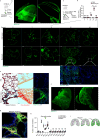
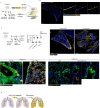
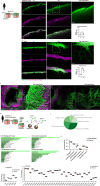
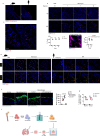
Lung fibrosis development utilizes alveolar macrophages, with mechanisms that are incompletely understood. Here, we fate map connective tissue during mouse lung fibrosis and observe disassembly and transfer of connective tissue macromolecules from pleuro-alveolar junctions (PAJs) into deep lung tissue, to activate fibroblasts and fibrosis. Disassembly and transfer of PAJ macromolecules into deep lung tissue occurs by alveolar macrophages, activating cysteine-type proteolysis on pleural mesothelium. The PAJ niche and the disassembly cascade is active in patient lung biopsies, persists in chronic fibrosis models, and wanes down in acute fibrosis models. Pleural-specific viral therapeutic carrying the cysteine protease inhibitor Cystatin A shuts down PAJ disassembly, reverses fibrosis and regenerates chronic fibrotic lungs. Targeting PAJ disassembly by targeting the pleura may provide a unique therapeutic avenue to treat lung fibrotic diseases.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare the following competing interests: A.F., M.M.H., S.K., and Y.R. have filed patent application EP21206 688.0 covering the use of these methods to study extracellular matrix movement in organ fibrosis. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Grants and funding
- 2016-01277/Fritz Thyssen Stiftung (Fritz Thyssen Foundation)
- K05 DA000017/DA/NIDA NIH HHS/United States
- ERC-CoG 819933/EC | EU Framework Programme for Research and Innovation H2020 | H2020 Priority Excellent Science | H2020 European Research Council (H2020 Excellent Science - European Research Council)
- CDA00017/2016/Human Frontier Science Program (HFSP)
- RI 2787/1-1 AOBJ: 628819/Deutsche Forschungsgemeinschaft (German Research Foundation)
LinkOut - more resources
Full Text Sources
Medical
Research Materials

