Deficiency in NPC2 results in disruption of mitochondria-late endosome/lysosomes contact sites and endo-lysosomal lipid dyshomeostasis
- PMID: 39747180
- PMCID: PMC11696400
- DOI: 10.1038/s41598-024-83460-x
Deficiency in NPC2 results in disruption of mitochondria-late endosome/lysosomes contact sites and endo-lysosomal lipid dyshomeostasis
Abstract
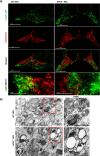
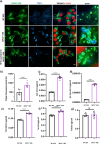
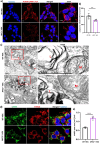
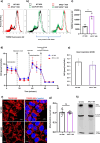
Dysfunction of the endo-lysosomal intracellular Cholesterol transporter 2 protein (NPC2) leads to the onset of Niemann-Pick Disease Type C (NPC), a lysosomal storage disorder. Metabolic and homeostatic mechanisms are disrupted in lysosomal storage disorders (LSDs) hence we characterized a cellular model of NPC2 knock out, to assess alterations in organellar function and inter-organellar crosstalk between mitochondria and lysosomes. We performed characterization of lipid alterations and confirmed altered lysosomal morphology, but no overt changes in oxidative stress markers. Using several techniques, we demonstrated that contacts between mitochondria and late endosomes/lysosomes are reduced in NPC2-/- HEK cells, we observed that the acidic compartments are swollen and lipid dense. Quantification of endogenous lipids in HEKNPC2-/- cells by mass spectrometry reveals accumulation of lipid species indicative of sphingolipid metabolic dysregulation within the lysosome. Specifically, HEK NPC2-/- cells exhibit marked elevation of glucosylsphingosine and glucosylceramides, substrates of beta glucocerebroside (GBA), as well as accumulation of sphingosine and sphingomyelins. Our studies suggest an involvement of NPC2 in the formation of contact sites between mitochondria and lysosomes and support the hypothesis of a role for NPC2 in the endo-lysosomal trafficking pathway and dynamic organellar crosstalk.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: Authors are employees of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Kenilworth, NJ, USA and may hold stocks and/or stock options in Merck & Co., Inc., Kenilworth, NJ, USA.
Figures
Similar articles
-
Targeting defective sphingosine kinase 1 in Niemann-Pick type C disease with an activator mitigates cholesterol accumulation.J Biol Chem. 2020 Jul 3;295(27):9121-9133. doi: 10.1074/jbc.RA120.012659. Epub 2020 May 8. J Biol Chem. 2020. PMID: 32385114 Free PMC article.
-
Finding pathogenic commonalities between Niemann-Pick type C and other lysosomal storage disorders: Opportunities for shared therapeutic interventions.Biochim Biophys Acta Mol Basis Dis. 2020 Oct 1;1866(10):165875. doi: 10.1016/j.bbadis.2020.165875. Epub 2020 Jun 6. Biochim Biophys Acta Mol Basis Dis. 2020. PMID: 32522631 Review.
-
Niemann-Pick C1 functions independently of Niemann-Pick C2 in the initial stage of retrograde transport of membrane-impermeable lysosomal cargo.J Biol Chem. 2010 Feb 12;285(7):4983-94. doi: 10.1074/jbc.M109.037622. Epub 2009 Dec 10. J Biol Chem. 2010. PMID: 20007703 Free PMC article.
-
Regulation of sterol transport between membranes and NPC2.Biochemistry. 2008 Oct 21;47(42):11134-43. doi: 10.1021/bi801328u. Epub 2008 Sep 30. Biochemistry. 2008. PMID: 18823126 Free PMC article.
-
Complex lipid trafficking in Niemann-Pick disease type C.J Inherit Metab Dis. 2015 Jan;38(1):187-99. doi: 10.1007/s10545-014-9794-4. Epub 2014 Nov 26. J Inherit Metab Dis. 2015. PMID: 25425283 Review.
References
-
- Platt, F. M., d’Azzo, A., Davidson, B. L., Neufeld, E. F. & Tifft, C. J. Lysosomal storage diseases. Nat. Rev. Disease Primers4, 27. 10.1038/s41572-018-0025-4 (2018). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

