TILTomorrow today: dynamic factors predicting changes in intracranial pressure treatment intensity after traumatic brain injury
- PMID: 39747195
- PMCID: PMC11696189
- DOI: 10.1038/s41598-024-83862-x
TILTomorrow today: dynamic factors predicting changes in intracranial pressure treatment intensity after traumatic brain injury
Abstract
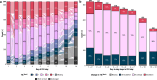
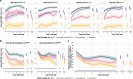
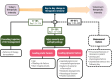
Practices for controlling intracranial pressure (ICP) in traumatic brain injury (TBI) patients admitted to the intensive care unit (ICU) vary considerably between centres. To help understand the rational basis for such variance in care, this study aims to identify the patient-level predictors of changes in ICP management. We extracted all heterogeneous data (2008 pre-ICU and ICU variables) collected from a prospective cohort (n = 844, 51 ICUs) of ICP-monitored TBI patients in the Collaborative European NeuroTrauma Effectiveness Research in TBI study. We developed the TILTomorrow modelling strategy, which leverages recurrent neural networks to map a token-embedded time series representation of all variables (including missing values) to an ordinal, dynamic prediction of the following day's five-category therapy intensity level (TIL(Basic)) score. With 20 repeats of fivefold cross-validation, we trained TILTomorrow on different variable sets and applied the TimeSHAP (temporal extension of SHapley Additive exPlanations) algorithm to estimate variable contributions towards predictions of next-day changes in TIL(Basic). Based on Somers' Dxy, the full range of variables explained 68% (95% CI 65-72%) of the ordinal variation in next-day changes in TIL(Basic) on day one and up to 51% (95% CI 45-56%) thereafter, when changes in TIL(Basic) became less frequent. Up to 81% (95% CI 78-85%) of this explanation could be derived from non-treatment variables (i.e., markers of pathophysiology and injury severity), but the prior trajectory of ICU management significantly improved prediction of future de-escalations in ICP-targeted treatment. Whilst there was no significant difference in the predictive discriminability (i.e., area under receiver operating characteristic curve) between next-day escalations (0.80 [95% CI 0.77-0.84]) and de-escalations (0.79 [95% CI 0.76-0.82]) in TIL(Basic) after day two, we found specific predictor effects to be more robust with de-escalations. The most important predictors of day-to-day changes in ICP management included preceding treatments, age, space-occupying lesions, ICP, metabolic derangements, and neurological function. Serial protein biomarkers were also important and may serve a useful role in the clinical armamentarium for assessing therapeutic needs. Approximately half of the ordinal variation in day-to-day changes in TIL(Basic) after day two remained unexplained, underscoring the significant contribution of unmeasured factors or clinicians' personal preferences in ICP treatment. At the same time, specific dynamic markers of pathophysiology associated strongly with changes in treatment intensity and, upon mechanistic investigation, may improve the timing and personalised targeting of future care.
Keywords: Data mining; Intensive care unit; Intracranial pressure; Machine learning; Therapy intensity level; Traumatic brain injury.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures


References
-
- Meyfroidt, G. et al. Management of moderate to severe traumatic brain injury: an update for the intensivist. Intensive Care Med.48, 649–666 (2022). - PubMed
-
- Maas, A. I. R. et al. Traumatic brain injury: Integrated approaches to improve prevention, clinical care, and research. Lancet Neurol.16, 987–1048 (2017). - PubMed
-
- Carney, N. et al. Guidelines for the management of severe traumatic brain injury, fourth edition. Neurosurgery80, 6 (2017). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

