Healthcare workers safety: a cohort study using healthcare utilisation databases on vaccination and vaccine timeliness impact against SARS-CoV-2 infection
- PMID: 39747281
- PMCID: PMC11695641
- DOI: 10.1038/s41598-024-84100-0
Healthcare workers safety: a cohort study using healthcare utilisation databases on vaccination and vaccine timeliness impact against SARS-CoV-2 infection
Abstract
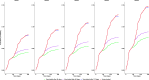
Healthcare Workers (HCWs) are at ongoing risk of SARS-CoV-2 infection, potentially contributing to its transmission. This study assessed full vaccination and vaccination timeliness impact on SARS-CoV-2 infections among HCWs in Italy's Marche Region, using Healthcare Utilization Databases. We evaluated vaccination coverage and its associated factors. The cohort comprised 21,118 HCWs aged 18-70 from the region's five Local Health Authorities (LHA), enrolled between February 2020 - May 2021. Factors associated with full vaccination were assessed using multiple logistic regression. The impact of vaccination status, time to vaccination, occupational role, age, gender, and health status on infection risk was analysed with a multiple Cox regression model, adjusting for vaccination coverage velocity, swabbing probability, and monthly intensive care unit admissions rate in each LHA. Of the cohort, 81.2% were fully vaccinated. Factors associated with full vaccination included age, role, LHA, prior infection, and health status. Vaccination reduced infection risk by 77% (95% CI: 70-82). Infection risk was higher among healthcare assistants, nurses/physiotherapists/technicians compared to physicians, among male HCWs, and it decreased as vaccination timeliness increased. Vaccination timeliness is crucial for reducing SARS-CoV-2 infection risk among HCWs, regardless of their characteristics. This underscores the importance of efficiently organizing vaccination administration across different territories and for all HCW categories.
Keywords: COVID-19; Healthcare Utilisation databases; Healthcare workers; SARS-CoV-2; Vaccine impact; Vaccine timeliness.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Due to the retrospective nature of the study, “Comitato Etico Territoriale (CET) delle Marche” waived the need of obtaining informed consent. According to the Italian privacy regulations (Legislation Decree 196/2003 amended by Legislation Decree 101/2018), studies using retrospective aggregated data from administrative databases that do not involve direct access by investigators to individual patients’ data, do not require approval or notification from an Ethics Committee/IRB. The data used in this research were anonymized and did not involve any personally identifiable information, ensuring compliance with the relevant legal frameworks for data protection and privacy. Competing interests: The authors declare no competing interests.
Figures
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

