Biochemical properties and substrate specificity of GOB-38 in Elizabethkingia anophelis
- PMID: 39747310
- PMCID: PMC11695579
- DOI: 10.1038/s41598-024-82748-2
Biochemical properties and substrate specificity of GOB-38 in Elizabethkingia anophelis
Abstract
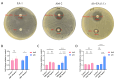
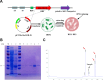
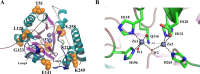
The novel pathogen, Elizabethkingia anophelis, has gained attention due to its high mortality rates and drug resistance facilitated by its inherent metallo-β-lactamases (MBLs) genes. This study successfully identified and outlined the functions of the B3-Q MBLs variant, GOB-38, in a clinical sample of E. anophelis. The T7 expression system was employed to stimulate the expression of recombinant protein in Escherichia coli, followed by an analysis of the biochemical properties of purified GOB-38. Our findings indicate that the enzyme GOB-38 displays a wide range of substrates, including broad-spectrum penicillins, 1-4 generation cephalosporins, and carbapenems, potentially contributing to in vitro drug resistance in E. coli through a cloning mechanism. It is important to highlight that GOB-38 exhibits a distinct active site composition compared to GOB-1/18, featuring hydrophilic amino acids Thr51 and Glu141 at both ends of its active center instead of hydrophobic alanine, potentially indicating a preference for imipenem. Furthermore, the co-isolation of Acinetobacter baumannii and E. anophelis, two opportunistic pathogens, from a single lung infection is noteworthy. Our in vitro co-culture experiments suggest that E. anophelis, carrying two MBL genes, may have the ability to transfer carbapenem resistance to other bacterial species through co-infection.
Keywords: Acinetobacter baumannii; Elizabethkingia anophelis; Antibiotic resistance; GOB-38; Metallo-β-lactamases.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethical approval: All research methods were approved by the Medical Ethics Committee of the First Affiliated Hospital of Nanchang University (CDYFYYLK 02–020) and were conducted in accordance with the Declaration of Helsinki and the legal requirements of the country (China) in which the work was performed. Informed consent was obtained from all participants.
Figures



Similar articles
-
In vitro activities of imipenem, vancomycin, and rifampicin against clinical Elizabethkingia species producing BlaB and GOB metallo-beta-lactamases.Eur J Clin Microbiol Infect Dis. 2019 Nov;38(11):2045-2052. doi: 10.1007/s10096-019-03639-3. Epub 2019 Jul 27. Eur J Clin Microbiol Infect Dis. 2019. PMID: 31352669
-
Crystal Structure of the Metallo-β-Lactamase GOB in the Periplasmic Dizinc Form Reveals an Unusual Metal Site.Antimicrob Agents Chemother. 2016 Sep 23;60(10):6013-22. doi: 10.1128/AAC.01067-16. Print 2016 Oct. Antimicrob Agents Chemother. 2016. PMID: 27458232 Free PMC article.
-
Molecular diversity of chromosomal metallo-β-lactamase genes in Elizabethkingia genus.Int J Antimicrob Agents. 2020 Jul;56(1):105978. doi: 10.1016/j.ijantimicag.2020.105978. Epub 2020 Apr 21. Int J Antimicrob Agents. 2020. PMID: 32325204
-
Carbapenem resistance in Acinetobacter baumannii: mechanisms and epidemiology.Clin Microbiol Infect. 2006 Sep;12(9):826-36. doi: 10.1111/j.1469-0691.2006.01456.x. Clin Microbiol Infect. 2006. PMID: 16882287 Review.
-
Screening and deciphering antibiotic resistance in Acinetobacter baumannii: a state of the art.Expert Rev Anti Infect Ther. 2013 Jun;11(6):571-83. doi: 10.1586/eri.13.38. Expert Rev Anti Infect Ther. 2013. PMID: 23750729 Review.
References
-
- Laxminarayan, R. et al. Antibiotic resistance-the need for global solutions. Lancet Infect. Dis.13, 1057–1098. 10.1016/s1473-3099(13)70318-9 (2013). - PubMed
-
- Gordon, N. C. & Wareham, D. W. Multidrug-resistant Acinetobacter baumannii: mechanisms of virulence and resistance. Int. J. Antimicrob. Agents. 35, 219–226. 10.1016/j.ijantimicag.2009.10.024 (2010). - PubMed
Publication types
MeSH terms
Substances
Supplementary concepts
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

