Peroxiredoxin 6 maintains mitochondrial homeostasis and promotes tumor progression through ROS/JNK/p38 MAPK signaling pathway in multiple myeloma
- PMID: 39747460
- PMCID: PMC11696808
- DOI: 10.1038/s41598-024-84021-y
Peroxiredoxin 6 maintains mitochondrial homeostasis and promotes tumor progression through ROS/JNK/p38 MAPK signaling pathway in multiple myeloma
Abstract
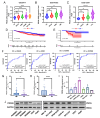
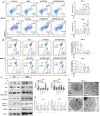
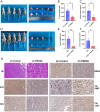
Peroxiredoxin 6 (PRDX6) is one of the Peroxiredoxin family members with only 1-Cys, using glutathione as the electron donor to reduce peroxides in cells. PRDX6 has been frequently studied and its expression was associated with poor prognosis in many tumors. However, the expression of PRDX6 in multiple myeloma (MM) and its relevance with MM remain unclear. In our study, we found that PRDX6 was overexpressed in MM patients. Its high expression was inversely correlated with prognosis but positively correlated with the levels of β2-microglobulin (B2M), lactate dehydrogenase (LDH), and International Staging System (ISS) stage of MM patients. Further, the deficiency of PRDX6 promoted MM cell lines (RPMI 8226, MM.1S, and U266) apoptosis significantly. Mechanically, PRDX6 serves as an anti-oxidative enzyme, and its deficiency led to over-accumulation of reactive oxygen species (ROS), resulting in oxidative stress, following the activation of MAPK signaling pathway, which manifested as phosphorylation of JNK and p38. Then, the expression of BAX and Bcl2 was imbalance, and the cascade cleavage of PARP and caspase 3 was increased, ultimately triggering cell apoptosis. In addition, oxidative stress decreased mitochondrial membrane potential (MMP), reduced gene expression levels of oxidative phosphorylation (OXPHOS), and increased in the density of mitochondrial crumpling, leading to mitochondrial structural abnormalities and dysfunction. Furthermore, PRDX6 deficiency combined with bortezomib induced a robust anti-tumor effect in MM cell lines. Finally, in vivo experiments also showed that the deficiency of PRDX6 inhibited tumor growth of tumor-bearing mice. Collectively, PRDX6 protects MM cells from oxidative damage and maintains mitochondrial homeostasis. And targeting PRDX6 is an attractive strategy to enhance the anti-tumor effect of bortezomib in MM.
Keywords: Apoptosis; Bortezomib; Mitochondrial homeostasis; Multiple myeloma; Reactive oxygen species.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures





Similar articles
-
PRDX6 Drives Breast Cancer Progression Through Mitochondrial Biosynthesis and Oxidative Phosphorylation.Cancer Med. 2025 Jul;14(13):e71005. doi: 10.1002/cam4.71005. Cancer Med. 2025. PMID: 40583735 Free PMC article.
-
BNIP3 overexpression may promote myeloma cell apoptosis by enhancing sensitivity to bortezomib via the p38 MAPK pathway.Hematology. 2023 Dec;28(1):2231739. doi: 10.1080/16078454.2023.2231739. Hematology. 2023. PMID: 37401850
-
Peroxiredoxin 6 Confers Protection Against Nonalcoholic Fatty Liver Disease Through Maintaining Mitochondrial Function.Antioxid Redox Signal. 2019 Aug 10;31(5):387-402. doi: 10.1089/ars.2018.7544. Epub 2019 May 22. Antioxid Redox Signal. 2019. PMID: 31007045
-
Anti-Oxidant and Pro-Oxidant Effects of Peroxiredoxin 6: A Potential Target in Respiratory Diseases.Cells. 2023 Jan 1;12(1):181. doi: 10.3390/cells12010181. Cells. 2023. PMID: 36611974 Free PMC article. Review.
-
Functional role of peroxiredoxin 6 in the eye.Free Radic Biol Med. 2018 Oct;126:210-220. doi: 10.1016/j.freeradbiomed.2018.08.017. Epub 2018 Aug 16. Free Radic Biol Med. 2018. PMID: 30120980 Review.
Cited by
-
Age-related declines in mitochondrial Prdx6 contribute to dysregulated muscle bioenergetics.Redox Biol. 2025 Aug 5;86:103808. doi: 10.1016/j.redox.2025.103808. Online ahead of print. Redox Biol. 2025. PMID: 40774144 Free PMC article.
-
HADHB mediates 5-fluorouracil sensitivity in colorectal cancer.Discov Oncol. 2025 Aug 28;16(1):1648. doi: 10.1007/s12672-025-03503-1. Discov Oncol. 2025. PMID: 40875107 Free PMC article.
-
Ameliorating effect of the aldose reductase inhibitor 1-Acetyl-5-phenyl-1 H-pyrrol-3-ylacetate on galactose-induced cataract.Sci Rep. 2025 Apr 14;15(1):12759. doi: 10.1038/s41598-025-98079-9. Sci Rep. 2025. PMID: 40229517 Free PMC article.
-
As2S2 Mediates the ROS/P38 MAPK Signaling Pathway to Induce Apoptosis and S-Phase Arrest in Myelodysplastic Syndrome Cells.Curr Issues Mol Biol. 2025 Apr 7;47(4):253. doi: 10.3390/cimb47040253. Curr Issues Mol Biol. 2025. PMID: 40699652 Free PMC article.
References
-
- Gandolfi, S. et al. The proteasome and proteasome inhibitors in multiple myeloma. Cancer Metastasis Rev.36(4), 561–584. 10.1007/s10555-017-9707-8 (2017). - PubMed
-
- Robak, P., Drozdz, I., Szemraj, J. & Robak, T. Drug resistance in multiple myeloma. Cancer Treat. Rev.70, 199–208. 10.1016/j.ctrv.2018.09.001 (2018). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

