A multicenter study on TROP2 as a potential targeted therapy for extramammary Paget disease in Japan
- PMID: 39747638
- PMCID: PMC11697375
- DOI: 10.1038/s41598-024-84566-y
A multicenter study on TROP2 as a potential targeted therapy for extramammary Paget disease in Japan
Abstract
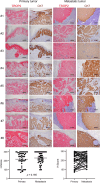
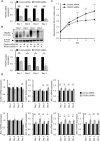
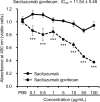
Extramammary Paget disease (EMPD) is a rare skin cancer that typically occurs in the anogenital area of older people. Since efficacy of treatments for metastatic or unresectable EMPD remains poor, development of a novel therapeutic approach is strongly desired. However, the lack of EMPD models has hampered investigation of EMPD. Here we investigated whether trophoblast cell surface antigen 2 (TROP2) could be a promising therapeutic target for EMPD. We retrospectively collected 108 samples from 54 patients with primary and metastatic EMPD from 10 Japanese institutions, and compared TROP2 expression between primary and metastatic lesions of each paired sample. In vitro assays were performed using a newly established EMPD cell line, KS-EMPD-1. TROP2 was strongly and homogeneously expressed in patient tissues, regardless of primary or metastatic lesions. The KS-EMPD-1 cells were treated with a TROP2-targeted antibody-drug conjugate (ADC), sacituzumab govitecan, and it significantly reduced cell viability in a dose-dependent manner compared with that of the cells treated with sacituzumab alone. Knockdown of TROP2 reduced cell viability and cell migration, and caused slight upregulation of the apoptosis-related factors, together with downregulation of the epithelial-to-mesenchymal transition-related factors. These findings suggest that a TROP2-targeted ADC may be a promising treatment option for unresectable EMPD.
Keywords: Antibody–drug conjugate; Extramammary Paget disease; Sacituzumab govitecan; Trophoblast cell surface antigen 2.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Trop2 Expression in Extramammary Paget's Disease and Normal Skin.Int J Mol Sci. 2021 Jul 19;22(14):7706. doi: 10.3390/ijms22147706. Int J Mol Sci. 2021. PMID: 34299325 Free PMC article.
-
TROP2 Expression and Therapeutic Implications in Cutaneous Squamous Cell Carcinoma: Insights From Immunohistochemical and Functional Analysis.Exp Dermatol. 2024 Oct;33(10):e15196. doi: 10.1111/exd.15196. Exp Dermatol. 2024. PMID: 39422290
-
NECTIN4-targeted antibody-drug conjugate is a potential therapeutic option for extramammary Paget disease.Exp Dermatol. 2024 Mar;33(3):e15049. doi: 10.1111/exd.15049. Exp Dermatol. 2024. PMID: 38509717
-
The Rise of the TROP2-Targeting Agents in NSCLC: New Options on the Horizon.Oncology. 2021;99(10):673-680. doi: 10.1159/000517438. Epub 2021 Jul 19. Oncology. 2021. PMID: 34280931 Review.
-
Evolution of TROP2: Biological insights and clinical applications.Eur J Med Chem. 2025 Oct 15;296:117863. doi: 10.1016/j.ejmech.2025.117863. Epub 2025 Jun 13. Eur J Med Chem. 2025. PMID: 40517574 Review.
References
-
- Ito, T., Kaku-Ito, Y. & Furue, M. The diagnosis and management of extramammary Paget’s disease. Expert Rev. Anticancer Ther.18, 543–553 (2018). - PubMed
-
- Hashimoto, H. & Ito, T. Current management and treatment of extramammary Paget’s disease. Curr. Treat. Options Oncol.23, 818–830 (2022). - PubMed
-
- Kanitakis, J. Mammary and extramammary Paget’s disease. J. Eur. Acad. Dermatol. Venereol.21, 581–590 (2007). - PubMed
-
- Ishida, Y. et al. Unbiased detection of driver mutations in extramammary Paget disease. Clin. Cancer Res.27, 1756–1765 (2021). - PubMed
-
- Iwasawa, O. et al. Mutational landscape of advanced extramammary Paget disease with comprehensive genomic profiling tests: A cohort study in a Japanese real-world setting. J. Am. Acad. Dermatol.90, 842–845 (2024). - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

