Combined endurance and resistance exercise training in heart failure with preserved ejection fraction: a randomized controlled trial
- PMID: 39747684
- PMCID: PMC11750725
- DOI: 10.1038/s41591-024-03342-7
Combined endurance and resistance exercise training in heart failure with preserved ejection fraction: a randomized controlled trial
Abstract
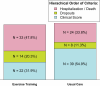
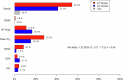
Endurance exercise training (ET) is an effective treatment in heart failure with preserved ejection fraction (HFpEF), but the efficacy of resistance training in this patient population has been only scarcely evaluated. In this multicenter, randomized trial, we evaluated the effects of combined endurance and resistance training over 12 months in patients with HFpEF. The primary endpoint was a modified Packer score, including all-cause mortality, hospitalizations classified as potentially related to heart failure or exercise and changes in peak oxygen consumption ( ), diastolic function (E/e'), New York Heart Association (NYHA) class and global self-assessment (GSA). In total, 322 patients (mean age, 70 years; 192 females (59.6%) and 130 males (40.4%)) were randomized (1:1) to ET or usual care (UC). At 12 months, the modified Packer score showed an improvement in 33 ET patients (20.5%) and in 13 UC patients (8.1%) and showed a worsening in 69 ET patients (42.9%) and in 71 UC patients (44.1%) (Kendall's tau-b = -0.073, P = 0.17). Although the primary endpoint was not met, clinically relevant differences favoring the ET group as compared to the UC group were observed for the following secondary endpoints: changes in peak (mean difference, 1.3 ml kg-1 min-1 (95% confidence interval (CI): 0.4-2.1)) and NYHA class (odds ratio = 7.77 (95% CI: 3.73-16.21)). No significant between-group differences were observed for other secondary endpoints, including change in E/e', change in GSA, time to cardiovascular hospitalization or all-cause mortality. In conclusion, 1 year of combined endurance and resistance ET did not result in a significantly better modified Packer score, but it did result in improvements in important clinical parameters, such as peak and NYHA class, as compared to UC. ISRCTN registration: ISRCTN86879094 .
© 2025. The Author(s).
Conflict of interest statement
Competing interests: F.E. reports personal fees from AstraZeneca, Bayer, Berlin Chemie, Boehringer Ingelheim, CVRx, Medtronic, Merck, Merck Sharp & Dohme, Novartis, Pfizer, PharmaCosmos, Resmed, Servier and Vifor Pharma; non-financial support from Novartis; and grants from AstraZeneca, Boehringer Ingelheim, Servier and Thermo Fisher Scientific, outside the submitted work. R.W. reports receiving personal fees from AstraZeneca, Bayer, Bristol Myers Squibb, Boehringer Ingelheim, CVRx, Daiichi Sankyo, Medtronic, Novartis, Pfizer, Pharmacosmos and Servier and research support from Boehringer Ingelheim, Bundesministerium für Bildung und Forschung, Deutsche Forschungsgemeinschaft, the European Union and Medtronic, outside the submitted work. S.M. reports personal fees from Bristol Myers Squibb (consulting services), outside the submitted work. E.P.-K. holds minor shares in ICTS GmbH (Imaging in Clinical Trials Services) and is a part-time employee of this company and reports receiving minor personal fees from AstraZeneca. H.-D.D. reports receiving personal fees from Bayer, Bristol Myers Squibb, Boehringer Ingelheim, Merck, Merck Sharp & Dohme, Novartis and Servier, outside the submitted work. H.-D.D. also holds shares in SCIRENT GmbH and d.o.o. (Clinical Trials Services). C.H.-L. reports a personal fee from Novartis; royalties from Hogrefe Publishing Group; and research grants from the German Ministry of Education and Research, the German Research Foundation and the EU Commission. K.E. reports personal fees from Bristol Myers Squibb and Boehringer Ingelheim (honoraria for lectures), outside the submitted work. A.H. reports personal fees from AstraZeneca, Boehringer Ingelheim, Bristol Myers Squibb, Cardiac Dimensions, Daiichi Sankyo, GE Healthcare and Novartis. G.H. reports personal fees from AstraZeneca, Bayer, Boehringer Ingelheim, Corvia, Impulse Dynamics, Novartis, Servier and Vifor Pharma; being a co-principal investigator for Impulse Dynamics; and business ownership of Avocet Bio GmbH. B.P. reports institutional grants from AstraZeneca, Bayer Healthcare and Boston Scientific and personal fees for steering committee, consulting and speaker services from Bayer Healthcare, Merck Sharp & Dohme, AstraZeneca, Boehringer Ingelheim, Novartis, Boston Scientific and Abbott, outside the submitted work. B.P. also holds minor shares in ICTS GmbH (Imaging in Clinical Trials Services). M.H. reports receiving personal fees from Abbott, Amgen, Boehringer Ingelheim, Bristol Myers Squibb, Daiichi Sankyo, Sanofi-Aventis, Novartis and Medical Park (consulting fees and honoraria for lectures) and being the past president of the European Association of Preventive Cardiology (2020–2022), outside the submitted work. No other potential conflicts of interest were reported. Additional collaborators: Stephan Gielen, Stephan von Haehling, Stefan Störk, Tobias D. Trippel, Stefan Anker, Hugo Saner, Mitja Lainscak, Andrea Berghold, Daniel Morris, Evgeny Belyavski, Martin Kropf and Aravind Kumar Radhakrishnan.
Figures








References
-
- Tsao, C. W. et al. Heart disease and stroke statistics—2023 update: a report from the American Heart Association. Circulation147, e93–e621 (2023). - PubMed
-
- Shah, K. S. et al. Heart failure with preserved, borderline, and reduced ejection fraction: 5-year outcomes. J. Am. Coll. Cardiol.70, 2476–2486 (2017). - PubMed
-
- Anker, S. D. et al. Empagliflozin in heart failure with a preserved ejection fraction. N. Engl. J. Med.385, 1451–1461 (2021). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

