Deep learning-based aberration compensation improves contrast and resolution in fluorescence microscopy
- PMID: 39747824
- PMCID: PMC11697233
- DOI: 10.1038/s41467-024-55267-x
Deep learning-based aberration compensation improves contrast and resolution in fluorescence microscopy
Abstract
Optical aberrations hinder fluorescence microscopy of thick samples, reducing image signal, contrast, and resolution. Here we introduce a deep learning-based strategy for aberration compensation, improving image quality without slowing image acquisition, applying additional dose, or introducing more optics. Our method (i) introduces synthetic aberrations to images acquired on the shallow side of image stacks, making them resemble those acquired deeper into the volume and (ii) trains neural networks to reverse the effect of these aberrations. We use simulations and experiments to show that applying the trained 'de-aberration' networks outperforms alternative methods, providing restoration on par with adaptive optics techniques; and subsequently apply the networks to diverse datasets captured with confocal, light-sheet, multi-photon, and super-resolution microscopy. In all cases, the improved quality of the restored data facilitates qualitative image inspection and improves downstream image quantitation, including orientational analysis of blood vessels in mouse tissue and improved membrane and nuclear segmentation in C. elegans embryos.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

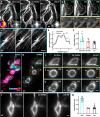
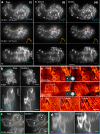
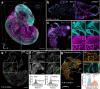
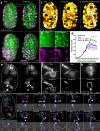
Update of
-
Deep learning-based aberration compensation improves contrast and resolution in fluorescence microscopy.bioRxiv [Preprint]. 2024 Jul 15:2023.10.15.562439. doi: 10.1101/2023.10.15.562439. bioRxiv. 2024. Update in: Nat Commun. 2025 Jan 2;16(1):313. doi: 10.1038/s41467-024-55267-x. PMID: 37986950 Free PMC article. Updated. Preprint.
References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources

