Dengue virus IgG and neutralizing antibody titers measured with standard and mature viruses are protective
- PMID: 39747846
- PMCID: PMC11697199
- DOI: 10.1038/s41467-024-53916-9
Dengue virus IgG and neutralizing antibody titers measured with standard and mature viruses are protective
Abstract
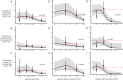
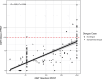
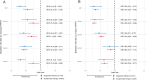
The standard dengue virus (DENV) neutralization assay inconsistently predicts dengue protection. We compare how IgG ELISA, envelope domain III (EDIII), or non-structural protein 1 (NS1) binding antibodies, and titers from plaque reduction neutralization tests (PRNTs) using standard and mature viruses are associated with dengue. The ELISA measures IgG antibodies that bind to inactivated DENV1-4. The EDIII and NS1 assays measure binding antibodies, and the PRNTs measure neutralizing antibodies to each specific DENV serotype. Healthy children (n = 1206) in Cebu, Philippines were followed for 5 years. ELISA IgG≥3 was associated with reduced dengue probability relative to naïve children (3% vs. 10%, p = 0.007). Serotype-specific antibodies binding EDIII or NS1 had no association with dengue risk. Standard virus PRNT geometric mean titers (GMT) > 200 and mature GMT > 100 were associated with reduced dengue disease overall (p < 0.01). High DENV2 and DENV3 titers against either standard or mature viruses protected against the matched serotype (p < 0.01). While 43% of dengue cases had standard virus PRNT titers>100, only 2% of cases had mature virus PRNT titers>100 (p < 0.001), indicating a lower, more consistent threshold for protection. These assays may serve as correlates of protection.
© 2024. This is a U.S. Government work and not under copyright protection in the US; foreign copyright protection may apply.
Conflict of interest statement
Competing interests: M.Y., M.V.C., J.V.D., K.A.A., A.M.C. and A.K.S. report receiving salaries from 2017 onwards as part of an ongoing separate study (effectiveness of the tetravalent dengue vaccine, CYD-TDV [Dengvaxia] in the Philippines) sponsored by the University of the Philippines Manila and funded by Sanofi Pasteur. J.D. was an unpaid external consultant in the Extended Study Group for dengue vaccine effectiveness evaluation studies in Asia in 2015 convened by Sanofi Pasteur and is an unpaid investigator of an ongoing separate study (effectiveness of the tetravalent dengue vaccine, CYD-TDV [Dengvaxia] in the Philippines) sponsored by the University of the Philippines Manila and funded by Sanofi Pasteur. The protocol was written to evaluate the effect of the Sanofi Pasteur Dengvaxia vaccine. However, only unvaccinated individuals were included in the present manuscript. All other authors declare no competing interests. Ethics: J.D., M.Y., J.V.D., M.V.C., and K.A.A. are investigators local to the Philippines who designed and established the cohort and collected all the demographic, clinical, and blood samples, and generated the enzyme-linked immunosorbent assay data. All local investigators were included in discussions of this work evaluating antibodies as correlates, roles, and responsibilities were agreed upon ahead of the research, and authorship was determined accordingly. This research is highly relevant to Cebu, Philippines, given the heavy local burden of disease, and this work was funded, in large part, by the Philippine Department of Health. Capacity building included additional training for JVD in the laboratory AMDS. This research did not result in stigmatization, incrimination, discrimination, or otherwise personal risk to participants. Related work by the local investigators has been cited appropriately here23,24.
Figures


Update of
-
Dengue virus IgG and serotype-specific neutralizing antibody titers measured with standard and mature viruses are associated with protection.Res Sq [Preprint]. 2024 Apr 12:rs.3.rs-4145863. doi: 10.21203/rs.3.rs-4145863/v1. Res Sq. 2024. Update in: Nat Commun. 2025 Jan 2;16(1):191. doi: 10.1038/s41467-024-53916-9. PMID: 38659845 Free PMC article. Updated. Preprint.
References
-
- WHO. WHO | Dengue guidelines, for diagnosis, treatment, prevention and control. http://www.who.int/neglected_diseases/resources/9789241547871/en/ (2009). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

