Wearable electrodriven switch actively delivers macromolecular drugs to fundus in non-invasive and controllable manners
- PMID: 39747871
- PMCID: PMC11695998
- DOI: 10.1038/s41467-024-55336-1
Wearable electrodriven switch actively delivers macromolecular drugs to fundus in non-invasive and controllable manners
Abstract
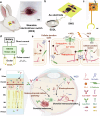
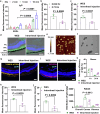
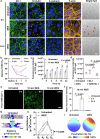
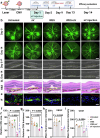
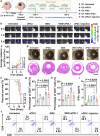
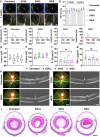
Current treatments for fundus disorders, such as intravitreal injections, pose risks, including infection and retinal detachment, and are limited in their ability to deliver macromolecular drugs across the blood‒retinal barrier. Although non-invasive methods are safer, their delivery efficiency remains suboptimal (<5%). We have developed a wearable electrodriven switch (WES) that improves the non-invasive delivery of macromolecules to the fundus. The WES system, which integrates an electrodriven drug delivery lens with a square wave generator, leverages electrical stimulation to enhance drug penetration through the sclera-choroid-retina pathway. In our study, WES achieved a delivery efficiency of 14% for immunoglobulin G, comparable to that of intravitreal injection (16%). Moreover, WES-enhanced anti-VEGF administration resulted in an 86% inhibition of choroidal neovascularization, and anti-PDL1 delivery inhibited choroidal melanoma growth more effectively than intravenous injections, with no adverse effects on ocular health. These findings suggest that WES holds transformative potential for the non-invasive treatment of chronic fundus diseases.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

