Comparison of oxidative stress status in the kidney tissue of male rats treated with paraquat and nanoparaquat
- PMID: 39747891
- PMCID: PMC11696234
- DOI: 10.1038/s41598-024-83156-2
Comparison of oxidative stress status in the kidney tissue of male rats treated with paraquat and nanoparaquat
Abstract
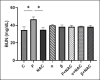
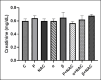
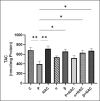
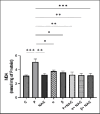
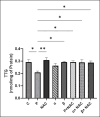
The study aimed to compare the oxidative stress status in the kidney tissue of rats treated with paraquat and nanoparaquat. The levels of oxidative stress markers, including malondialdehyde (MDA), total antioxidant capacity (TAC), and thiol groups (TTG), were measured in the kidney tissue samples. A total of forty male Wistar rats were randomly assigned to eight groups, each consisting of five rats: a control group, a paraquat (PQ) group, an N-acetylcysteine (NAC) group, groups receiving nanoparaquat α and β (α and β), groups receiving PQ and NAC (PQ + NAC), and groups receiving nanoparaquat α and β with NAC (+ NACα and β). Paraquat, a widely used herbicide, induces severe oxidative damage in kidneys through radical formation and cellular stress. Newly developed nanoparaquat formulations may modify its toxicity profile and tissue distribution patterns. The results revealed that rats treated with paraquat showed a significant increase in Lipid Peroxidation Oxidation (LPO) levels compared to the control group and those treated with NAC. However, treatment with nanoparaquat α and β resulted in a decrease in LPO levels compared to the paraquat-treated group. Additionally, when nanoparaquat α and β were administered in combination with NAC, a further reduction in LPO levels was observed compared to the PQ treated group. Regarding TAC levels, the PQ group exhibited a significant decrease compared to the control group and the NAC-treated group. However, treatment with nanoparaquat β resulted in higher TAC levels compared to the PQ group. Moreover, when nanoparaquat α and β were administered in combination with NAC, there was an increase in TAC levels compared to the PQ group. In terms of TTG levels, the PQ group showed a significant decrease compared to the control group and the NAC group. However, treatment with nanoparaquat β led to an increase in TTG levels compared to the PQ group. Furthermore, when nanoparaquat α and β were administered in combination with NAC, there was an increase in TTG levels compared to the PQ group. Overall, the results suggest that treatment with nanoparaquat, especially nanoparaquat β, may have a protective effect against oxidative stress induced by PQ toxicity in the kidney tissue of rats. Further studies are warranted to elucidate the underlying mechanisms and potential therapeutic implications of nanoparaquat in oxidative stress-related kidney disorders.
Keywords: Chitosan; Kidney; Nanoparticles; Oxidative Stress; Paraquat; Rats.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Conflict of interest: The authors declare that they have no known competing financial or non-financial interests that could have appeared to influence the work reported in this paper. Animal Ethical Considerations and Informed Consent: All protocols in this study were approved by the Committee on the Ethics of Animal Experiments of Hamadan University of Medical Sciences Ethics Committee on Animal Care, Hamadan, Iran (IR.UMSHA.REC.1400.631), in compliance with the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH publication no.85 − 23, revised 1996). All experimental procedures and reporting follow the ARRIVE guidelines for animal research.
Figures

References
-
- Pasi, A. The toxicity of paraquat, diquat and morfamquat. Vienna: Hans Huber. 13 – 5. (1978).
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

