Phthalocyanine aggregates as semiconductor-like photocatalysts for hypoxic-tumor photodynamic immunotherapy
- PMID: 39747902
- PMCID: PMC11696155
- DOI: 10.1038/s41467-024-55575-2
Phthalocyanine aggregates as semiconductor-like photocatalysts for hypoxic-tumor photodynamic immunotherapy
Abstract
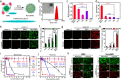
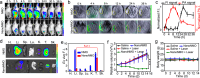
Photodynamic immunotherapy (PIT) has emerged as a promising approach for efficient eradication of primary tumors and inhibition of tumor metastasis. However, most of photosensitizers (PSs) for PIT exhibit notable oxygen dependence. Herein, a concept emphasizing on transition from molecular PSs into semiconductor-like photocatalysts is proposed, which converts the PSs from type II photoreaction to efficient type I photoreaction. Detailed mechanism studies reveal that the nanostructured phthalocyanine aggregate (NanoNMe) generates radical ion pairs through a photoinduced symmetry breaking charge separation process, achieving charge separation through a self-substrate approach and leading to exceptional photocatalytic charge transfer activity. Additionally, a reformed phthalocyanine aggregate (NanoNMO) is fabricated to improve the stability in physiological environments. NanoNMO showcases significant photocytotoxicities under both normoxic and hypoxic conditions and exhibits remarkable tumor targeting ability. Notably, the NanoNMO-based photodynamic therapy and PD-1 checkpoint inhibitor-based immunotherapy synergistically triggers the infiltration of cytotoxic T lymphocytes into the tumor sites of female mice, leading to the effective inhibition of breast tumor growth.
© 2025. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures






Similar articles
-
Oncolytic reovirus enhances the effect of CEA immunotherapy when combined with PD1-PDL1 inhibitor in a colorectal cancer model.Immunotherapy. 2025 Apr;17(6):425-435. doi: 10.1080/1750743X.2025.2501926. Epub 2025 May 12. Immunotherapy. 2025. PMID: 40353308
-
A Hypoxia-Triggered Bioreduction of Hydrophilic Type I Photosensitizer for Switchable In Vivo Photoacoustic Imaging and High-Specificity Cancer Phototherapy.Angew Chem Int Ed Engl. 2025 Jun 17;64(25):e202506412. doi: 10.1002/anie.202506412. Epub 2025 Apr 17. Angew Chem Int Ed Engl. 2025. PMID: 40204649 Free PMC article.
-
Blackberry-Like Doxorubicin Loaded Hyaluronic Acid/Zinc Phthalocyanine Loaded Mesoporous Silica Nanocomposites for Long-Term Tumor Photodynamic and Chemotherapy Synergistic Therapy.Int J Nanomedicine. 2025 Jul 17;20:9111-9134. doi: 10.2147/IJN.S530041. eCollection 2025. Int J Nanomedicine. 2025. PMID: 40692537 Free PMC article.
-
Recent advances in type I organic photosensitizers for efficient photodynamic therapy for overcoming tumor hypoxia.J Mater Chem B. 2023 May 31;11(21):4600-4618. doi: 10.1039/d3tb00545c. J Mater Chem B. 2023. PMID: 37183673 Review.
-
Systemic treatments for metastatic cutaneous melanoma.Cochrane Database Syst Rev. 2018 Feb 6;2(2):CD011123. doi: 10.1002/14651858.CD011123.pub2. Cochrane Database Syst Rev. 2018. PMID: 29405038 Free PMC article.
References
-
- Felsher, D. W. Cancer revoked: Oncogenes as therapeutic targets. Nat. Rev. Cancer3, 375–380 (2003). - PubMed
-
- Brown, J. M. & Wilson, W. R. Exploiting tumour hypoxia in cancer treatment. Nat. Rev. Cancer4, 437–447 (2004). - PubMed
-
- Li, X., Lovell, J. F., Yoon, J. & Chen, X. Clinical development and potential of photothermal and photodynamic therapies for cancer. Nat. Rev. Clin. Oncol.17, 657–674 (2020). - PubMed
-
- Dolmans, D., Fukumura, D. & Jain, R. K. Photodynamic therapy for cancer. Nat. Rev. Cancer3, 380–387 (2003). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

