Physiological premature aging of ovarian blood vessels leads to decline in fertility in middle-aged mice
- PMID: 39747922
- PMCID: PMC11695630
- DOI: 10.1038/s41467-024-55509-y
Physiological premature aging of ovarian blood vessels leads to decline in fertility in middle-aged mice
Abstract
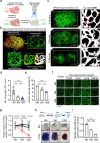
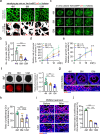
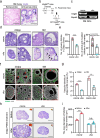
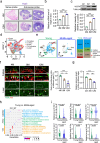
Ovarian function declines significantly as females enter middle-age, but the mechanisms underlying this decline remain unclear. Here, we utilize whole-organ imaging to observe a notable decrease in ovarian blood vessel (oBV) density and angiogenesis intensity of middle-aged mice. This leads to a diminished blood supply to the ovaries, resulting in inadequate development and maturation of ovarian follicles. Utilizing genetic-modified mouse models, we demonstrate that granulosa cell secreted VEGFA governs ovarian angiogenesis, but the physiological decline in oBV is not attributed to VEGFA insufficiency. Instead, through single-cell sequencing, we identify the aging of the ovarian vascular endothelium as the primary factor contributing to oBV decline. Consequently, the administration of salidroside, a natural compound that is functional to reverse oBV aging and promote ovarian angiogenesis, significantly enhances ovarian blood supply and improve fertility in older females. Our findings highlight that enhancing oBV function is a promising strategy to boost fertility in females.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The author declares no competing interests.
Figures

References
-
- Adams, O. Life expectancy in Canada-an overview. Health Rep.2, 361–376 (1990). - PubMed
-
- Li, J. et al. Determining a multimodal aging clock in a cohort of Chinese women. Med.4, 825–848.e813 (2023). - PubMed
-
- te Velde, E. R. & Pearson, P. L. The variability of female reproductive ageing. Hum. Reprod. Update8, 141–154 (2002). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

