Multi-omic biomarker panel in pancreatic cyst fluid and serum predicts patients at a high risk of pancreatic cancer development
- PMID: 39747972
- PMCID: PMC11696309
- DOI: 10.1038/s41598-024-83742-4
Multi-omic biomarker panel in pancreatic cyst fluid and serum predicts patients at a high risk of pancreatic cancer development
Abstract
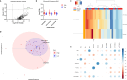
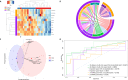
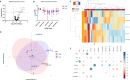
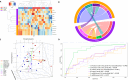
Integration of multi-omic data for the purposes of biomarker discovery can provide novel and robust panels across multiple biological compartments. Appropriate analytical methods are key to ensuring accurate and meaningful outputs in the multi-omic setting. Here, we extensively profile the proteome and transcriptome of patient pancreatic cyst fluid (PCF) (n = 32) and serum (n = 68), before integrating matched omic and biofluid data, to identify biomarkers of pancreatic cancer risk. Differential expression analysis, feature reduction, multi-omic data integration, unsupervised hierarchical clustering, principal component analysis, spearman correlations and leave-one-out cross-validation were performed using RStudio and CombiROC software. An 11-feature multi-omic panel in PCF [PIGR, S100A8, REG1A, LGALS3, TCN1, LCN2, PRSS8, MUC6, SNORA66, miR-216a-5p, miR-216b-5p] generated an AUC = 0.806. A 13-feature multi-omic panel in serum [SHROOM3, IGHV3-72, IGJ, IGHA1, PPBP, APOD, SFN, IGHG1, miR-197-5p, miR-6741-5p, miR-3180, miR-3180-3p, miR-6782-5p] produced an AUC = 0.824. Integration of the strongest performing biomarkers generated a 10-feature cross-biofluid multi-omic panel [S100A8, LGALS3, SNORA66, miR-216b-5p, IGHV3-72, IGJ, IGHA1, PPBP, miR-3180, miR-3180-3p] with an AUC = 0.970. Multi-omic profiling provides an abundance of potential biomarkers. Integration of data from different omic compartments, and across biofluids, produced a biomarker panel that performs with high accuracy, showing promise for the risk stratification of patients with pancreatic cystic lesions.
Keywords: Biomarker; Multi-omics; Pancreatic cancer; Pancreatic cystic lesion; Risk stratification.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Ethics approval and consent to participate: This work was performed in accordance with the Code of Ethics of the World Medical Association (Declaration of Helsinki) for experiments involving humans. Patients provided informed consent for sample and data acquisition, and the study received full ethical approval from Tallaght University Hospital Joint Research Ethics Committee Review Board (ID: 0319-264).
Figures




Similar articles
-
An integrated multi-omics biomarker approach using molecular profiling and microRNAs for evaluation of pancreatic cyst fluid.Cancer Cytopathol. 2025 Apr;133(4):e70008. doi: 10.1002/cncy.70008. Cancer Cytopathol. 2025. PMID: 40106268
-
Next generation sequencing of pancreatic cyst fluid microRNAs from low grade-benign and high grade-invasive lesions.Cancer Lett. 2015 Jan 28;356(2 Pt B):404-9. doi: 10.1016/j.canlet.2014.09.029. Epub 2014 Oct 7. Cancer Lett. 2015. PMID: 25304377 Free PMC article.
-
Plasma microRNA panels to diagnose pancreatic cancer: Results from a multicenter study.Oncotarget. 2016 Jul 5;7(27):41575-41583. doi: 10.18632/oncotarget.9491. Oncotarget. 2016. PMID: 27223429 Free PMC article.
-
Proteomics analysis of bodily fluids in pancreatic cancer.Proteomics. 2015 Aug;15(15):2705-15. doi: 10.1002/pmic.201400476. Epub 2015 Apr 27. Proteomics. 2015. PMID: 25780901 Free PMC article. Review.
-
Pancreatic Cyst Fluid Analysis.Gastrointest Endosc Clin N Am. 2023 Jul;33(3):599-612. doi: 10.1016/j.giec.2023.03.006. Epub 2023 Apr 25. Gastrointest Endosc Clin N Am. 2023. PMID: 37245938 Review.
References
-
- Siegel, R. L., Giaquinto, A. N. & Jemal, A. Cancer statistics, 2024. CA Cancer J. Clin.74(1), 12–49 (2024). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

