FSTL1 aggravates high glucose-induced oxidative stress and transdifferentiation in HK-2 cells
- PMID: 39748077
- PMCID: PMC11696259
- DOI: 10.1038/s41598-024-84462-5
FSTL1 aggravates high glucose-induced oxidative stress and transdifferentiation in HK-2 cells
Abstract
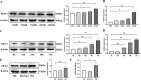
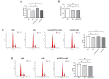
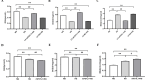
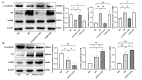
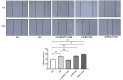
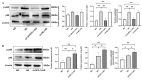
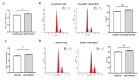
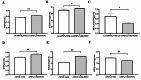
Chronic hyperglycemia, a hallmark of diabetes, can trigger inflammatory responses in the kidney, leading to diabetic nephropathy (DN). Follistatin-like protein 1 (FSTL1) has emerged as a potential therapeutic target in various kidney diseases. This study investigated the effect of high glucose on FSTL1 expression and its role in oxidative stress and cellular transdifferentiation injury in HK-2 human proximal tubule epithelial cells, a model of DN. We investigated FSTL1's level in HK-2 cells exposed to high glucose using Western blotting and quantitative real-time polymerase chain reaction (qRT-PCR). FSTL1 was manipulated using recombinant human FSTL1 (rhFSTL1) or lentiviral shFSTL1. We then analyzed proliferation, oxidative stress, transdifferentiation, cell migration, and the nuclear factor kappa-B (NF-κB) signaling pathway potentially involved in FSTL1 effects. Finally, we blocked the NF-κB pathway to see its influence on these cellular processes. High glucose exposure significantly increased FSTL1 in HK-2 cells, with longer/higher glucose further amplifying this effect. Silencing of FSTL1 ameliorates cellular damage by promoting proliferation, enhancing superoxide dismutase (SOD) and glutathione (GSH) activity, and reducing malondialdehyde (MDA) production, inhibiting cell migration. Furthermore, it prevented the harmful conversion of HK-2 cells from epithelial to myofibroblast-like phenotypes, evidenced by decreased fibronectin (FN) and α-smooth muscle actin (α-SMA) and preserved E-cadherin. Notably, silencing FSTL1 also inhibited the NF-κB signaling pathway. Conversely, rhFSTL1 exhibited opposite effects. Importantly, blocking NF-κB reversed the detrimental effects of FSTL1. These findings suggest that FSTL1 contributes to high glucose-induced kidney injury by promoting oxidative stress and cellular transdifferentiation potentially via the NF-κB pathway. Targeting FSTL1 may represent a novel therapeutic strategy for preventing or mitigating DN progression.
Keywords: Diabetic nephropathy; Follistatin-like protein 1; Oxidative stress; Transdifferentiation.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures

References
-
- Zhang, Y. & Xia, Y. Role of follistatin-like 1 (Fstl1) in chronic kidney disease. Kidney Int. Rep.4, S387 (2019). - DOI
Publication types
MeSH terms
Substances
Grants and funding
- No. Sz2013-003/Jiamusi University Key Project, China
- No. JMSUGPZR2023-019/Jiamusi University National Foundation Cultivation Program, China
- No. ZHY2024-104/Chinese Medicine Research Program in Heilongjiang Province, China
- No. 2022-KYYWF-0656/Heilongjiang Provincial Higher Education Institutions' Basic Research Operating Expenses Project, China
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

