Protective effect of compound K against podocyte injury in chronic kidney disease by maintaining mitochondrial homeostasis
- PMID: 39748100
- PMCID: PMC11696807
- DOI: 10.1038/s41598-024-84704-6
Protective effect of compound K against podocyte injury in chronic kidney disease by maintaining mitochondrial homeostasis
Abstract
Chronic kidney disease (CKD) stands as a formidable global health challenge, often advancing to end-stage renal disease (ESRD) with devastating morbidity and mortality. At the central of this progression lies podocyte injury, a critical determinant of glomerular dysfunction. Compound K (CK), a bioactive metabolite derived from ginsenoside, has emerged as a compelling candidate for nephroprotective therapy. Here, we unveil the profound therapeutic potential of CK in a folic acid (FA)-induced CKD mouse model, demonstrating its ability to restore renal function and mitigate podocyte injury. CK exerted its nephroprotective effects by reinforcing inter-podocyte junctions, suppressing aberrant podocyte motility, and preventing podocyte detachment and apoptosis, thereby safeguarding the glomerular filtration barrier. Mechanistically, we identified mitochondrial dysregulation as a key driver of excessive oxidative stress, which is commonly associated with podocyte damage. CK remarkably restored mitochondrial homeostasis by attenuating pathological mitochondrial fission and enhancing mitophagy, thereby rebalancing the delicate mitochondrial network. Intriguingly, CK may disrupt the formation of the Drp1-Bax dimer, a crucial mediator of mitochondrial apoptosis, further averting podocyte loss. Collectively, our findings highlight CK as a potent nephroprotective agent, offering a novel therapeutic avenue for CKD management and redefining possibilities in the battle against progressive renal disease.
Keywords: Apoptosis; Chronic kidney disease; Compound K; Mitochondrial homeostasis; Podocyte Injury.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare no competing interests. Declaration of competing interest: The authors declare that they have no conflicts of interest. Ethics statement: All experiments involving animals have been approved by the Institutional Animal Care and Use Committee of Zhejiang Chinese Medical University (the approval number of ethics committee: IACUC-20240429-16). All animal procedures were adhered to internationally accepted standards for animal research, following the 3Rs principle. The ARRIVE guidelines were employed for reporting experiments involving live animals, promoting ethical research practices.
Figures
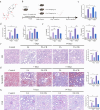

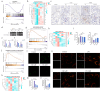

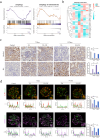

References
Publication types
MeSH terms
Substances
Grants and funding
- No. 2022YKJ03/the Zhejiang Chinese Medicine University Postgraduate Scientific Research Fund Project
- No. 81803980/Discipline Construction Project of Zhejiang Chinese Medical University, the National Natural Science Foundation of China
- No. 2022JKZZW03/the Research Project of Zhejiang Chinese Medical University
- 2020ZG09/the Research Project of Zhejiang Chinese Medical University
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

