Silencing circ_0043256 inhibited CoCl2-induced proliferation, migration, and aerobic glycolysis in gastric cancer cells
- PMID: 39748101
- PMCID: PMC11697268
- DOI: 10.1038/s41598-024-84548-0
Silencing circ_0043256 inhibited CoCl2-induced proliferation, migration, and aerobic glycolysis in gastric cancer cells
Abstract
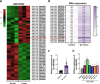
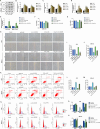
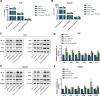
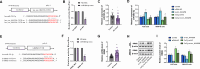
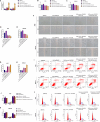
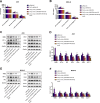
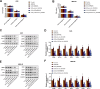
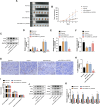
We aimed to explore the role of circular RNA 0043256 (circ_0043256) in gastric cancer (GC) and its underlying mechanisms. The impact of circ_0043256 silencing on the proliferation, migration, apoptosis, and aerobic glycolysis of MKN-45 and AGS cells induced by CoCl2 was assessed through the utilization of CCK-8, wound healing assay, flow cytometry, and metabolic analysis. The interaction between circ_0043256 and miR-593-5p, as well as the involvement of the miR-593-5p/RRM2 axis in gastric cancer, were confirmed via luciferase assay, Western blot, and bioinformatics analysis. We found that circ_0043256 was up-regulated in GC tissues and CoCl2-treated MKN-45 and AGS cells. Silencing of circ_0043256 reversed CoCl2-induced proliferation, migration, and aerobic glycolysis in MKN-45 and AGS cells. Additionally, circ_0043256 silencing enhanced cell apoptosis and G2/M phase cell cycle arrest in response to CoCl2 treatment. Furthermore, the miR-593-5p/RRM2 axis was identified as a regulatory mechanism for circ_0043256 function in GC. Silencing of circ_0043256 and miR-593-5p mimic co-transfection significantly inhibited CoCl2-induced cellular responses in MKN-45 and AGS cells. A glycolysis inhibitor 2-DG further enhanced the inhibitory effect of circ_0043256 silencing on aerobic glycolysis of CoCl2-induced MKN-45 and AGS cells. Additionally, the inhibition of circ_0043256 resulted in a reduction in tumor volume and the expression of proliferation marker proteins in nude mice. Moreover, the suppression of circ_0043256 led to an increase in miR-593-5p expression and a decrease in RRM2 expression, ultimately causing a decrease in glycolytic-related proteins associated with the glycolytic pathway. Targeting this axis may offer a novel therapeutic approach for treating GC.
Keywords: Aerobic glycolysis; Circ_0043256; Gastric cancer; RRM2; miR-593-5p.
© 2024. The Author(s).
Conflict of interest statement
Declartions. Competing interests: The authors declare no competing interests. Approval of the research protocol by an institutional reviewer board: The study protocols were approved by the Experimental Animal Ethics Committee, West China Hospital, Sichuan University (20240617005). Informed consent: All specimens were collected after obtaining informed consent from all patients.
Figures

Similar articles
-
The Circular RNA Circ_0043947 Promoted Gastric Cancer Progression by Sponging miR-384 to Regulate CREB1 Expression.Gut Liver. 2024 Nov 15;18(6):977-991. doi: 10.5009/gnl230173. Epub 2024 Apr 19. Gut Liver. 2024. PMID: 38638101 Free PMC article.
-
Hsa_circ_0017639 expression promotes gastric cancer proliferation and metastasis by sponging miR-224-5p and upregulating USP3.Gene. 2020 Aug 5;750:144753. doi: 10.1016/j.gene.2020.144753. Epub 2020 May 4. Gene. 2020. PMID: 32376451
-
Circular RNA Circ_0079226 Plays an Oncogenic Role in Gastric Cancer via the miR-155-5p/FOXK1/AKT Pathway.Anal Cell Pathol (Amst). 2025 Feb 13;2025:6619550. doi: 10.1155/ancp/6619550. eCollection 2025. Anal Cell Pathol (Amst). 2025. PMID: 39981141 Free PMC article.
-
hsa_circ_001653 up-regulates NR6A1 expression and elicits gastric cancer progression by binding to microRNA-377.Exp Physiol. 2020 Dec;105(12):2141-2153. doi: 10.1113/EP088399. Epub 2020 Nov 13. Exp Physiol. 2020. PMID: 33006200
-
miR-449c-5p availability is antagonized by circ-NOTCH1 for MYC-induced NOTCH1 upregulation as well as tumor metastasis and stemness in gastric cancer.J Cell Biochem. 2020 Oct;121(10):4052-4063. doi: 10.1002/jcb.29575. Epub 2020 Jan 14. J Cell Biochem. 2020. PMID: 31943342
Cited by
-
Circular RNAs as Targets for Developing Anticancer Therapeutics.Cells. 2025 Jul 18;14(14):1106. doi: 10.3390/cells14141106. Cells. 2025. PMID: 40710359 Free PMC article. Review.
References
-
- Smyth, E. C., Nilsson, M., Grabsch, H. I., van Grieken, N. C. & Lordick, F. Gastric cancer. Lancet (London, England)29 (10251), 635–648. 10.1016/s0140-6736(20)31288-5 (2020). - PubMed
-
- Takahashi, K. & Yoshikawa, T. Different risks of nodal metastasis by tumor location in remnant gastric cancer after curative gastrectomy for gastric cancer. Gastric Cancer23 (1), 195–201. 10.1007/s10120-019-00989-x (2020). - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

