Exosome-derived long non-coding RNA AC010789.1 modified by FTO and hnRNPA2B1 accelerates growth of hair follicle stem cells against androgen alopecia by activating S100A8/Wnt/β-catenin signalling
- PMID: 39748192
- PMCID: PMC11695201
- DOI: 10.1002/ctm2.70152
Exosome-derived long non-coding RNA AC010789.1 modified by FTO and hnRNPA2B1 accelerates growth of hair follicle stem cells against androgen alopecia by activating S100A8/Wnt/β-catenin signalling
Abstract
Background: The increased incidence of androgenic alopecia (AGA) causes adverse physiological and psychological effects on people of all genders. The hair follicle stem cells (HFSCs) have displayed clinical improvements on AGA. However, the molecular mechanism of HFSCs against AGA remains elusive.
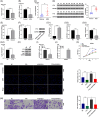
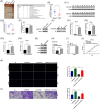
Methods: The expression and prognosis of lncRNA AC010789.1 in AGA hair follicle tissues were assessed by qRT-PCR analysis. CCK-8, EdU and Transwell analysis were utilized to assess cell growth. The specific binding between AC010789.1 and FTO mediated m6A modification or the effect of AC010789.1 on hnRNPA2B1, S100A8 and Wnt/β-catenin signaling expression was confirmed by bioinformatic analysis, RIP, RNA pull-down and Western blot assay. The effects of Exosome-loaded AC010789.1 prompted HFSCs proliferation and hair follicle regeneration were confirmed in hairless mice.
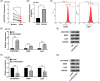
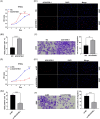
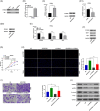
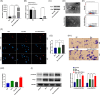
Results: We herein found that the mRNA levels of lncRNA AC010789.1 were decreased in AGA tissue samples but increased in HFSCs of surrounding normal tissue samples. Overexpression (OE) of AC010789.1 promoted HFSC proliferation, DNA synthesis and migration as well as K6HF and Lgr5 upregulation, whereas knockdown of AC010789.1 showed the opposite effects. The total or AC010789.1 m6A levels were reduced and FTO demethylase was upregulated in AGA tissue samples, but these indicated the reverse results in HFSCs of surrounding normal tissue samples. FTO OE decreased AC010789.1 m6A levels and its mRNA levels in HFSCs and abolished AC010789.1-induced HFSCs proliferation. In addition, AC010789.1 was identified to bind to m6A reader hnRNPA2B1, which was downregulated in AGA but upregulated in HFSCs of surrounding normal tissue samples. hnRNPA2B1 OE attenuated AC010789.1 knockdown-induced inhibition of HFSCs proliferation. Moreover, AC010789.1 could bind to and enhance downstream S100A8 protein expression, which mediated Wnt/β-catenin signaling to accelerate HFSCs proliferation. Exosome-loaded AC010789.1 prompted HFSCs proliferation and hair follicle regeneration in mice.
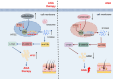
Conclusions: Our findings demonstrated that exosome-derived lncRNA AC010789.1 modified by FTO and hnRNPA2B1 facilitated the proliferation of human HFSCs against AGA by activating S100A8/Wnt/β-catenin signaling.
Key points: Long non-coding RNA (lncRNA) AC010789.1 was downregulated in hair follicle tissues from androgenic alopecia (AGA) and upregulated in hair follicle stem cells (HFSCs). LncRNA AC010789.1 promoted the proliferation and migration of HFSCs. FTO/hnRNPA2B1-mediated m6A modification of lncRNA AC010789.1 promoted HFSCs growth by activating S100A8/Wnt/β-catenin signalling. Exosome-derived AC010789.1 accelerated HFSCs proliferation.
Keywords: FTO; HFSCs; S100A8; hnRNPA2B1; lncRNA AC010789.1; proliferation.
© 2025 The Author(s). Clinical and Translational Medicine published by John Wiley & Sons Australia, Ltd on behalf of Shanghai Institute of Clinical Bioinformatics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

References
-
- Piraccini BM, Alessandrini A. Androgenetic alopecia. G Ital Dermatol Venereol. 2014;149(1):15‐24. - PubMed
-
- Leirós GJ, Ceruti JM, Castellanos ML, et al. Androgens modify Wnt agonists/antagonists expression balance in dermal papilla cells preventing hair follicle stem cell differentiation in androgenetic alopecia. Mol Cell Endocrinol. 2017;439:26‐34. - PubMed
-
- Dong C, Du J, Yu Z, et al. Global research status and trends in hair follicle stem cells: a bibliometric analysis. Stem Cell Rev Rep. 2022;18(6):2002‐2015. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous
