Hydroxychloroquine enhances insulin sensitivity and ameliorates abnormal lipid metabolism in obese women with polycystic ovary syndrome
- PMID: 39748269
- PMCID: PMC11697925
- DOI: 10.1186/s12902-024-01827-7
Hydroxychloroquine enhances insulin sensitivity and ameliorates abnormal lipid metabolism in obese women with polycystic ovary syndrome
Abstract
Background: Hydroxychloroquine (HCQ) is frequently utilized in rheumatic immune disorders and has been discovered to exert hypoglycemic effects in some obese women with polycystic ovary syndrome(PCOS), however, the precise efficacy and mechanism of action remain ambiguous.
Objective: To examine the impact of HCQ on glucose and lipid metabolism as well as sex hormone levels in obese women with PCOS.
Method: Fifty obese women with PCOS were randomly allocated into two groups: HCQ group (n = 25) and metformin (MET) group (n = 25). The HCQ group received a daily dose of 200 mg hydroxychloroquine, while the MET group received a daily dose of 1000 mg metformin. Body fat parameters, glucose and lipid metabolism levels, as well as hormone levels were evaluated. Additionally, the incidence of pregnancy within six months following treatment was also assessed. Network pharmacology was also employed to analyze the potential molecular mechanism.
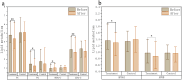
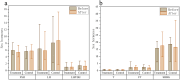
Result: Patients in the HCQ group (n = 20) and MET group (n = 23) were ultimately included for analysis. Following treatment, both groups exhibited significant improvements in body fat distribution and glucose metabolism status, with the HCQ group demonstrating a notable advantage over the MET group in increasing insulin sensitivity index (ISI)(HCQ:1.87 ± 0.21,MET:1.75 ± 0.29). Serum lipid levels [Serum total cholesterol(TC, mmol/L)(HCQ:4.51 ± 0.87,MET:5.05 ± 0.65), triglyceride(TG, mmol/L)(HCQ:1.36 ± 0.51,MET:1.67 ± 0.72), low-density lipoprotein (LDL, mmol/L)(HCQ:2.66 ± 0.98,MET:0.47 ± 1.42),decreased in both groups post-treatment, with the HCQ group displaying clear advantages compared to the MET group. The improvement of sex hormone levels was not pronounced in either group, although there was an overall downward trend.
Conclusion: The potential benefits of HCQ in the management of in obese women with PCOS include significant improvements in body fat distribution, glucose and lipid metabolism levels, as well as correction of hormonal disorders.
Clinical trial registration: The study was officially registered as a clinical trial on April 17, 2022, with the registration number ChiCTR2200058816. https://www.chictr.org.cn/showproj.html?proj=160099 .
Keywords: Hydroxychloroquine; Insulin resistance; Lipid metabolism; Polycystic ovary syndrome.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethical approval: This study was performed in line with the principles of the Declaration of Helsinki. The research protocol has been approved by the Ethics Committee of the Reproductive and Genetic Center of Shandong University of Traditional Chinese Medicine (2021 Clinical Research Application No. 17). Informed consent was obtained from all individual participants included in the study. Consent for publication: Informed consent was obtained from all individual participants included in the study. Identifying images or personal or clinical details of other participants presented in this article are anonymous, so this statement does not applicable. Statements& declaration: The main findings in the paper were independently conducted by the author or the research team. It is important to avoid plagiarism and properly cite the work of other authors. Additionally, there were no instances of submitting the same work to multiple publications or presenting it at different conferences. CONSORT guidelines: This manuscript is in line with the CONSORT clinical trial reporting guidelines, and we have uploaded the CONSORT guidelines as attachments. Competing interests: The authors declare no competing interests.
Figures





References
-
- Balen AH, Morley LC, Misso M, Franks S, Legro RS, Wijeyaratne CN, et al. The management of anovulatory infertility in women with polycystic ovary syndrome: an analysis of the evidence to support the development of global WHO guidance. Hum Reprod Update. 2016;22(6):687-708. 10.1093/humupd/dmw025. Epub 2016/08/12. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

