Integration of transcriptome and metabolome reveals key regulatory defense pathways associated with high temperature stress in cucumber (Cucumis sativus L.)
- PMID: 39748295
- PMCID: PMC11694469
- DOI: 10.1186/s12870-024-05876-x
Integration of transcriptome and metabolome reveals key regulatory defense pathways associated with high temperature stress in cucumber (Cucumis sativus L.)
Abstract
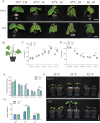
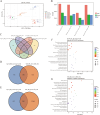
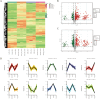
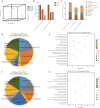
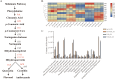
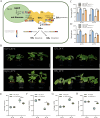
High temperature stress seriously affects the quality and yield of vegetable crops, especially cucumber (Cucumis sativus L.). However, the metabolic dynamics and gene regulatory network of cucumber in response to high temperature stress remain poorly studied. In this study, we identified a heat-tolerant cucumber Gy14 and a heat-sensitive cucumber 32X. RNA-seq analysis of Gy14 and 32X under high temperature stress showed that some differentially expressed genes (DEGs) were related to the biosynthesis of secondary metabolites. Metabolomic analysis revealed that there were more phenylpropanoids and their downstream derivatives in Gy14 compared to that in 32X under Re_2d condition (2 normal days recovery after heat). Integrated analysis of transcriptome and metabolome revealed that these upregulated genes played a pivotal role in flavonoid biosynthesis. Moreover, high temperature stress significantly induced the expression of the gibberellin (GA) biosynthesis genes and exogenous application of GA3 alleviated the damage of high temperature to cucumber seedlings. Together, these findings provided new insights into the transcriptome response and metabolomic reprogramming of cucumber against high temperature stress.
Keywords: Cucumber; Flavonoids; High temperature stress; Metabolome; Transcriptome.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The cucumber seeds used in this study are stored in our laboratory and are commonly used experimental materials. The methods involved in this study were carried out in compliance with local and national regulations. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

