Reduction of pain and functional disability over time in patients treated with zavegepant: a post-hoc analysis of the BHV3500-301 phase 3 randomized controlled trial
- PMID: 39748312
- PMCID: PMC11696797
- DOI: 10.1186/s10194-024-01915-y
Reduction of pain and functional disability over time in patients treated with zavegepant: a post-hoc analysis of the BHV3500-301 phase 3 randomized controlled trial
Abstract
Background: Migraine is a disabling disorder that impacts 40 million people in the US. Zavegepant is the first calcitonin gene-related peptide (CGRP) receptor antagonist nasal-spray approved for the acute treatment of migraine with or without aura in adults. This study aimed to evaluate the proportion of patients in various pain and functional disability states over 48-h, for patients treated with zavegepant 10 mg nasal-spray versus placebo.
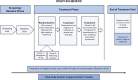
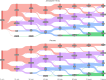
Methods: This post-hoc analysis included adult patients with > 1-year history of migraine from BHV3500-301 (NCT04571060): a phase 3 double-blind, randomized, placebo-controlled, single-attack study. Over 48-h, pain severity and functional disability were captured at various timepoints (pre- and post-dosing). The proportion of patients at each pain severity or functional disability state and the time spent in each category was calculated. These were analyzed for patients with complete timepoint data available and using missing not at random (MNAR) imputation for missing timepoints. Predictors of functional disability were assessed using a mixed-effects logistic regression model.
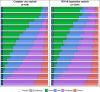
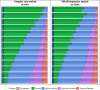
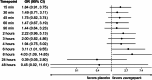
Results: There were 1,269 patients included in the MNAR imputation analysis, and between 630-641 in the complete-case analysis. As early as 15-min post-dose, a larger proportion of zavegepant patients achieved no/mild pain compared to placebo, despite balanced migraine severity pre-dose. Furthermore, zavegepant patients spent significantly more time (over 2.5-h) in pain freedom compared to placebo. Similarly, a higher proportion of patients with normal function was observed with zavegepant vs placebo, as early as 30-min post-dose. Over 48-h, patients treated with zavegepant spent an average of ~ 3-h longer with normal functioning compared to placebo. Results were similar when analyzing both analytic groups. In a regression model, treatment with zavegepant, lower pain severity, fewer baseline monthly migraine days, and absence of photophobia, phonophobia, and nausea were associated with better functioning (p < 0.05) over 48-h.
Conclusion: This post-hoc analysis demonstrates the benefit of zavegepant nasal spray over placebo on two patient-centric endpoints: time spent with pain freedom and normal functioning over 48-h post-dose. These data support the use of zavegepant for providing rapid and sustained freedom from migraine pain and freedom from migraine related disability, particularly for those who would benefit from the nasal CGRP formulation.
Keywords: Calcitonin gene-related peptide; Functional disability; Migraine; Pain; Randomized controlled trial; Therapeutics.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approvals and consent to participate: Ethics approval for the BHV3500-301 (NCT04571060) trial was approved was obtained in accordance with the principles of the Guidelines for Good Clinical Practice, the Declaration of Helsinki, and all applicable local regulations. The protocol was approved by a central institutional review board (Advarra IRB, Columbia, MD, USA, number 0000080) and by a local institutional review board (Biomedical Research Alliance of New York Institutional Review Board, Lake Success, NY, USA, number 00010793) for one study centre. Consent for publication: No applicable. Competing interests: BR, FOS, LP, and PJ, and are employees of Broadstreet HEOR, which received funds from Pfizer for this work. FD JC, LA and SS are employed by and own stock/stock options in Pfizer. JA has received personal compensation for serving as a consultant for Pfizer.
Figures


References
-
- Migraine research foundation. Migraine research 2019; https://migraineresearchfoundation.org/about-migraine/migraine-facts/. Accessed July 8, 2020.
-
- GBD 2016 Disease and Injury Incidence and Prevalence Collaborators (2017) Global, regional, and national incidence, prevalence, and years lived with disability for 328 diseases and injuries for 195 countries, 1990–2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet 390(10100):1211–1259 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

