Developing and assessing the "MultiLife" intervention: a mobile health-based lifestyle toolkit for cardiometabolic multimorbidity in diabetes and hypertension management - a type 1 hybrid effectiveness-implementation trial protocol
- PMID: 39748357
- PMCID: PMC11694374
- DOI: 10.1186/s12889-024-20922-x
Developing and assessing the "MultiLife" intervention: a mobile health-based lifestyle toolkit for cardiometabolic multimorbidity in diabetes and hypertension management - a type 1 hybrid effectiveness-implementation trial protocol
Abstract
Background: Cardiometabolic multimorbidity (CMM), characterized by the coexistence of diabetes, hypertension, and cardiovascular disease, poses a major health challenge in India, particularly in rural areas with limited healthcare resources. Lifestyle interventions can manage cardiometabolic risk factors, yet adherence remains suboptimal. Mobile health (mHealth) interventions offer a scalable approach for managing CMM by promoting behaviour change and medication adherence. We will develop and evaluate the MultiLife intervention, a mHealth-based lifestyle toolkit aimed at improving CMM management among individuals receiving primary care in Eastern India in the year 2025.
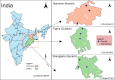
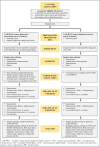
Methods: This study is a two-arm, cluster-randomized controlled trial with a hybrid Type 1 design involving 840 participants across 18 primary health centres in Odisha and Jharkhand. Using the Health Belief Model as a conceptual framework, the MultiLife intervention will deliver daily digital reminders, weekly health education broadcasts, and ongoing primary care support in the intervention arm, while the control group will receive the standard ongoing primary care support care. The trained healthcare workers will recruit 50 CMM patients, with a 6-month intervention period, during routine visits in each cluster. Primary outcomes include changes in HbA1c from baseline (T0) to end-line (T6). Secondary outcomes include blood pressure, body mass index, physical activity, and dietary habits. Qualitative assessments will explore intervention barriers and facilitators. Implementation outcomes, assessed through the RE-AIM QuEST framework, will evaluate MultiFrame's acceptability, adoption, fidelity, and maintenance. A random-effects regression model will be used for difference-in-difference analysis, adjusting for covariates and within-cluster correlations.
Discussion: The MultiLife trial may provide valuable insights into how mHealth-enabled primary care can enhance patient engagement, adherence, and cardiovascular risk reduction in resource-constrained settings. By integrating patient perspectives, this study could inform scalable digital health strategies for comprehensive CMM management, providing a model for future interventions in similar contexts.
Trial registration: CTRI.nic.in, CTRI/2024/10/074559, Registered on 1 October 2024.
Keywords: Healthy lifestyle; India; Multimorbidity; Primary health care; Randomized controlled trial; Rural health; Technology transfer; Telemedicine.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Ethical clearance has been granted by Institutional ethics committees, ICMR Regional Medical Research Centre for (ICMR-RMRC/IHEC-2024/026), Institutional Ethics Committee, RIMS, Ranchi ((ECR/769/INST/JH/2015/RR-21/Letter no: 259) and State ethics committee, Odisha (22629/MS-2-IV-01/2024). Before engaging in any phase of this research, all study participants will be required to provide informed consent. The research will be conducted in compliance with the applicable guidelines and regulations outlined in the Declaration of Helsinki. Competing interests: The authors declare no competing interests.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical