Co-housing with Tibetan chickens improved the resistance of Arbor Acres chickens to Salmonella enterica serovar Enteritidis infection by altering their gut microbiota composition
- PMID: 39748400
- PMCID: PMC11697627
- DOI: 10.1186/s40104-024-01132-2
Co-housing with Tibetan chickens improved the resistance of Arbor Acres chickens to Salmonella enterica serovar Enteritidis infection by altering their gut microbiota composition
Abstract
Background: Salmonella enterica serovar Enteritidis (S. Enteritidis) is a global foodborne pathogen that poses a significant threat to human health, with poultry being the primary reservoir host. Therefore, addressing S. Enteritidis infections in poultry is crucial to protect human health and the poultry industry. In this study, we investigated the effect of co-housing Arbor Acres (AA) chickens, a commercial breed susceptible to S. Enteritidis, with Tibetan chickens, a local breed resistant to S. Enteritidis infection, on the resistance of the latter to the pathogen.
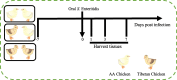
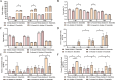
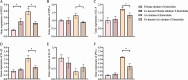
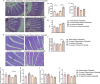
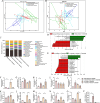
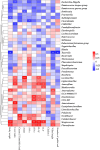
Results: Ninety-six 1-day-old Tibetan chickens and 96 1-day-old AA chickens were divided into a Tibetan chicken housed alone group (n = 48), an AA chicken housed alone group (n = 48), and a co-housed group (48 birds from each breed for 2 cages). All birds were provided the same diet, and the experimental period lasted 14 d. At d 7, all chickens were infected with S. Enteritidis, and samples were collected at 1-, 3-, and 7-day-post-infection. We found that the body weight of AA chickens significantly increased when co-housed with Tibetan chickens at 1- and 3-day-post-infection (P < 0.05). In addition, the cecal S. Enteritidis load in AA chickens was significantly reduced at 1-, 3-, and 7-day-post-infection (P < 0.05). Furthermore, the inflammatory response in AA chickens decreased, as evidenced by the decreased expression of pro-inflammatory cytokines NOS2, TNF-α, IL-8, IL-1β, and IFN-γ in their cecal tonsils (P < 0.05). Co-housing with Tibetan chickens significantly increased the height of villi and number of goblet cells (P < 0.05), as well as the expression of claudin-1 (P < 0.05), a tight junction protein, in the jejunum of AA chickens. Further analysis revealed that co-housing altered the gut microbiota composition in AA chickens; specifically, the relative abundances of harmful microbes, such as Intestinimonas, Oscillibacter, Tuzzerella, Anaerotruncus, Paludicola, and Anaerofilum were reduced (P < 0.05).
Conclusions: Our findings indicate that co-housing with Tibetan chickens enhanced the resistance of AA chickens to S. Enteritidis infection without compromising the resistance of Tibetan chickens. This study provides a novel approach for Salmonella control in practical poultry production.
Keywords: Salmonella enterica serovar Enteritidis; Arbor Acres chicken; Co-housing; Gut microbiota; Tibetan chicken.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: All animal experiments were conducted in accordance with the Regulations of the Experimental Animal Administration issued by the State Committee of Science and Technology of the People’s Republic of China, approved by the Animal Care and Use Committee of the Poultry Institute, Chinese Academy of Agriculture Science (approval No. CNP20220410). Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
Figures
References
-
- Wibisono FM, Wibison FJ, Effendi MH, Plumeriastuti H, Hidayatullah AR, Hartadi EB, et al. A review of salmonellosis on poultry farms: Public health importance. Sys Rev Pharm. 2020;11(9):481–6.
Grants and funding
LinkOut - more resources
Full Text Sources

