FOSL1 transcriptionally dictates the Warburg effect and enhances chemoresistance in triple-negative breast cancer
- PMID: 39748430
- PMCID: PMC11697476
- DOI: 10.1186/s12967-024-06014-9
FOSL1 transcriptionally dictates the Warburg effect and enhances chemoresistance in triple-negative breast cancer
Abstract
Background: Dysregulated energy metabolism has emerged as a defining hallmark of cancer, particularly evident in triple-negative breast cancer (TNBC). Distinct from other breast cancer subtypes, TNBC exhibits heightened glycolysis and aggressiveness. However, the transcriptional mechanisms of aerobic glycolysis in TNBC remains poorly understood.
Methods: The Cancer Genome Atlas (TCGA) cohort was utilized to identify genes associated with glycolysis. The role of FOSL1 in glycolysis and tumor growth in TNBC cells was confirmed through both loss-of-function and gain-of-function experiments. The subcutaneous xenograft model was established to evaluate the therapeutic potential of targeting FOSL1 in TNBC. Additionally, chromatin immunoprecipitation and luciferase reporter assays were employed to investigate the transcriptional regulation of glycolytic genes mediated by FOSL1.
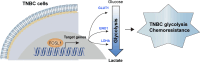
Results: FOSL1 is identified as a pivotal glycolysis-related transcription factor in TNBC. Functional verification shows that FOSL1 enhances the glycolytic metabolism of TNBC cells, as evidenced by glucose uptake, lactate production, and extracellular acidification rates. Notably, FOSL1 promotes tumor growth in TNBC in a glycolysis-dependent manner, as inhibiting glycolysis with 2-Deoxy-D-glucose markedly diminishes the oncogenic effects of FOSL1 in TNBC. Mechanistically, FOSL1 transcriptionally activates the expression of genes such as SLC2A1, ENO1, and LDHA, which further accelerate the glycolytic flux. Moreover, FOSL1 is highly expressed in doxorubicin (DOX)-resistant TNBC cells and clinical samples from cases of progressive disease following neoadjuvant chemotherapy. Targeting FOSL1 proves effective in overcoming chemoresistance in DOX-resistant MDA-MB-231 cells.
Conclusion: In summary, FOSL1 establishes a robust link between aerobic glycolysis and carcinogenesis, positioning it as a promising therapeutic target, especially in the context of TNBC chemotherapy.
Keywords: Drug resistance; Energy metabolism; Gene promoter; Glucose metabolism; Glucose transporter.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study received approval from the Research Ethics Committee of The First Hospital of Jilin University. All patients involved in this study signed informed consent. Consent for publication: All authors agree to submit the article for publication. Competing interests: The authors declare that they have no competing interests.
Figures



References
-
- Leon-Ferre RA, Goetz MP. Advances in systemic therapies for triple negative breast cancer. Bmj-British Med J 2023;381. - PubMed
-
- Chen CH, Huang YM, Grillet L, et al. Gallium maltolate shows synergism with cisplatin and activates nucleolar stress and ferroptosis in human breast carcinoma cells. Cell Oncol. 2023;46:1127–42. - PubMed
-
- Sharma P. Time to optimize deescalation strategies in Triple-negative breast Cancer? Clin Cancer Res. 2022;28:4840–2. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

