Glutathione reductase modulates endogenous oxidative stress and affects growth and virulence in Avibacterium paragallinarum
- PMID: 39748435
- PMCID: PMC11697956
- DOI: 10.1186/s13567-024-01388-6
Glutathione reductase modulates endogenous oxidative stress and affects growth and virulence in Avibacterium paragallinarum
Erratum in
-
Correction: Glutathione reductase modulates endogenous oxidative stress and affects growth and virulence in Avibacterium paragallinarum.Vet Res. 2025 Mar 21;56(1):61. doi: 10.1186/s13567-025-01499-8. Vet Res. 2025. PMID: 40119370 Free PMC article. No abstract available.
Abstract
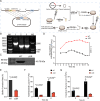
Glutathione reductase (GR) plays a pivotal role in managing oxidative stress, a process crucial for microbial virulence and adaptation, yet it has not been extensively explored in bacteria such as Avibacterium paragallinarum (Av. paragallinarum). This study examined the specific roles of GR in Av. paragallinarum, focusing on how GR modulates the bacterium's response to oxidative stress and impacts its pathogenic behavior. Using gene knockouts together with transcriptomic and metabolomic profiling, we identified an important shift in redox balance due to GR deficiency, which disrupted energy metabolism and weakened the oxidative stress defense, culminating in a notable decline in virulence. In addition, decreased growth rates, reduced biofilm production, and weakened macrophage interactions were observed in GR-deficient strains. Notably, our findings reveal a sophisticated adaptation mechanism wherein the bacterium recalibrated its metabolic pathways in response to GR deficiency without fully restoring virulence. Our in vivo studies further highlight the pivotal role of GR in pathogen fitness. Together, our findings connect GR-mediated redox control to bacterial virulence, thereby furthering the understanding of microbial adaptation and positioning GR as a potential antimicrobial target. Our insights into the GR-centric regulatory network pave the way for leveraging bacterial redox mechanisms in the development of novel antimicrobial therapies, highlighting the importance of oxidative stress management in bacterial pathogenicity.
Keywords: Av. paragallinarum; Bacterial virulence; Glutathione reductase; Metabolic adaptation; Oxidative stress; Redox homeostasis.
© 2025. The Author(s).
Conflict of interest statement
Declarations. Competing interests: The authors declare that they have nocompeting interests
Figures




References
-
- Blackall PJ, Christensen H, Beckenham T, Blackall LL, Bisgaard M (2005) Reclassification of Pasteurella gallinarum, [Haemophilus] paragallinarum, Pasteurella avium and Pasteurella volantium as Avibacterium gallinarum gen. nov., comb. nov., Avibacterium paragallinarum comb. nov., Avibacterium avium comb. nov. and Avibacterium volantium comb. nov. Int J Syst Evol Microbiol 55:353–362 - PubMed
-
- Schmidt-Brauns J, Herbert M, Kemmer G, Kraiss A, Schlör S, Reidl J (2001) Is a NAD pyrophosphatase activity necessary for Haemophilus influenzae type b multiplication in the blood stream? Int J Med Microbiol 291:219–225 - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

