Monitoring for advanced disease in the universal test and treat era: trends in CD4 count testing in South Africa
- PMID: 39748443
- PMCID: PMC11694356
- DOI: 10.1186/s44263-024-00118-6
Monitoring for advanced disease in the universal test and treat era: trends in CD4 count testing in South Africa
Abstract
Background: Under South Africa's Universal Test and Treat (UTT) policy, CD4 counts are no longer required to determine HIV treatment eligibility. However, CD4 count at presentation remains an important marker of disease progression. We assessed whether CD4 testing declined in the UTT era and, if so, by how much.
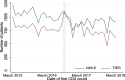
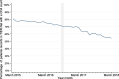
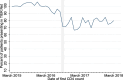
Methods: We analysed CD4 count data from the National Health Laboratory Service (NHLS) National HIV Cohort and TIER.Net database for individuals in HIV care across five South African provinces. "First CD4 count" was defined as the first CD4 test recorded for each patient. In TIER.Net, "date of presentation" was the earliest date of HIV testing, CD4 measurement, or clinic visit. Trends in first CD4 testing volumes (2004-2018) were analyzed, with interrupted time-series analyses assessing the impact of UTT (September 2016).
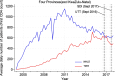
Results: Data included 5,274,218 (NHLS) and 2,265,557 (TIER.Net) individuals with a first CD4 count. In NHLS, first CD4 counts increased from 47,604 in 2004 to 383,705 in 2010 and then declined. Lower volumes were recorded in TIER.Net. Adjusting for prior trends, first CD4 counts increased slightly after UTT, by 32 individuals/day in NHLS (95% CI: - 6 to 61) and 88 individuals/day in TIER.Net (95% CI: 30 to 148). Among TIER.Net patients, the percentage with a CD4 count decreased by 4.3% (95% CI: - 5.2 to - 3.0%).
Conclusions: We found no major decline in CD4 testing at presentation following UTT, contrasting findings from resource-constrained settings with greater reliance on external donors.
Keywords: Advanced HIV disease; CD4 count testing; Interrupted time-series analysis; Trends in HIV care; Universal Test and Treat (UTT).
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Human Research Ethics Committee (HREC) of the University of Witwatersrand (ref. no. M200447) in South Africa and Boston University in the USA (IRB no. H-31968). No study participant recruitment took place since all data were collected as part of routine care in both databases. Data access for anonymized patient laboratory records in TIER.Net and NHLS were obtained from provincial departments of health as well as the NHLS. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures
References
-
- World Health Organization. Consolidated guidelines on the use of antiretro_viral drugs for treating and preventing HIV infection: recommendations for a public health approach.le. Vol. 2nd ed. Geneva; 2016. Available from: https://www.who.int/hiv/pub/arv/arv-2016/en/ - PubMed
-
- South African National Department of Health (South Africa). Implementation of Universal Test and Treat strategy for HIV positive patients and differentiated care for stable patients. Pretoria; 2016.
-
- Boulle A, Schomaker M, May MT, Hogg RS, Shepherd BE, Monge S, et al. Mortality in patients with HIV-1 infection starting antiretroviral therapy in South Africa, Europe, or North America: a collaborative analysis of prospective studies. PLoS Med. 2014;11(9)e1001718. 10.1371/journal.pmed.1001718. - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
