Physiologically Based Pharmacokinetic Modeling of Cannabidiol, Delta-9-Tetrahydrocannabinol, and Their Metabolites in Healthy Adults After Administration by Multiple Routes
- PMID: 39748462
- PMCID: PMC11695271
- DOI: 10.1111/cts.70119
Physiologically Based Pharmacokinetic Modeling of Cannabidiol, Delta-9-Tetrahydrocannabinol, and Their Metabolites in Healthy Adults After Administration by Multiple Routes
Abstract
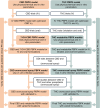
The two most extensively studied cannabinoids, cannabidiol (CBD) and delta-9-tetrahydrocannabinol (THC), are used for myriad conditions. THC is predominantly eliminated via the cytochromes P450 (CYPs), whereas CBD is eliminated through both CYPs and UDP-glucuronosyltransferases (UGTs). The fractional contributions of these enzymes to cannabinoid metabolism have shown conflicting results among studies. Physiologically based pharmacokinetic (PBPK) models for CBD and THC and for drug-drug interaction studies involving CBD or THC as object drugs were developed and verified to improve estimates of these contributions. First, physicochemical and pharmacokinetic parameters for CBD, THC, and their metabolites (7-OH-CBD, 11-OH-THC, and 11-COOH-THC) were obtained from the literature or optimized. Second, PBPK base models were developed for CBD and THC after intravenous administration. Third, beginning with the intravenous models, absorption models were developed for CBD after oral and oromucosal spray administration and for THC after oral, inhalation, and oromucosal spray administration. The full models well-captured the area under the concentration-time curve (AUC) and peak concentration (Cmax) of CBD and THC from the verification dataset. Predicted AUC and Cmax for CBD and 7-OH-CBD were within two-fold of the observed data. For THC, 11-OH-THC, and 11-COOH-THC, 100%, 100%, and 83% of the predicted AUC values were within two-fold, respectively, of the observed values; 100%, 92%, and 94% of the predicted Cmax values, respectively, were within two-fold of the observed values. The verified models could be used to help address critical public health needs, including assessing potential drug interaction risks involving CBD and THC.
Keywords: PBPK; cannabidiol; cannabinoids; delta‐9‐tetrahydrocannabinol; metabolites; pharmacokinetics.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
J.D. is an employee of Simcyp Ltd/Certara, a company that provides PBPK Software. M.F.P. is a member of the Scientific Advisory Board for Simcyp Ltd/Certara. All other authors declared no competing interests for this work.
Figures

References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

