Population Pharmacokinetic-Pharmacodynamic Modeling of Granulocyte Colony-Stimulating Factor to Optimize Dosing and Timing for CD34+ Cell Harvesting
- PMID: 39748466
- PMCID: PMC11695272
- DOI: 10.1111/cts.70121
Population Pharmacokinetic-Pharmacodynamic Modeling of Granulocyte Colony-Stimulating Factor to Optimize Dosing and Timing for CD34+ Cell Harvesting
Abstract
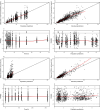
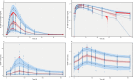
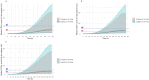
Granulocyte colony-stimulating factor (G-CSF) mobilizes peripheral blood (PB) progenitor cells from bone marrow (BM) into circulation for PB stem cell transplantation (PBSCT). This study aimed to develop a population pharmacokinetic-pharmacodynamic (PK-PD) model of filgrastim in healthy subjects to optimize PB CD34+ cell collection. Plasma filgrastim concentrations and CD34+ cell count data were obtained from a clinical study involving healthy Korean subjects. A total of 1378 plasma concentration measurements and 982 CD34+ cell count data collected from 53 subjects were used in the PK-PD model. Filgrastim PKs were adequately described by a one-compartment linear disposition model with an additional transit compartment for absorption. Log-transformed body weight was the only significant covariate affecting the volume of distribution and clearance. CD34+ cell mobilization was best captured by a modified Friberg model, assuming continual entry of proliferating BM stem cells into PB via a single transit compartment. Simulation results suggested that the 5 μg/kg twice-daily dosing regimen may yield higher CD34+ cell counts compared to the 10 μg/kg once-daily regimen for achieving target CD34+ cell counts of 20/μL and 50/μL. We successfully developed a robust PK-PD model of G-CSF that optimizes the yield of CD34+ cells during allogeneic PBSCT. This model can guide the efficient determination of optimal G-CSF dosing regimens and CD34+ cell harvesting strategies.
Keywords: CD34+; filgrastim; granulocyte colony‐stimulating factor; population pharmacokinetic‐pharmacodynamic modeling.
© 2025 The Author(s). Clinical and Translational Science published by Wiley Periodicals LLC on behalf of American Society for Clinical Pharmacology and Therapeutics.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures

References
-
- Yu X., Liu L., Xie Z., et al., “Bone Marrow Versus Peripheral Blood as a Graft Source for Haploidentical Donor Transplantation in Adults Using Post‐Transplant Cyclophosphamide‐A Systematic Review and Meta‐Analysis,” Critical Reviews in Oncology/Hematology 133 (2019): 120–128. - PubMed
-
- Yang S. H., Wang T. F., Tsai H. H., Lin T. Y., Wen S. H., and Chen S. H., “Preharvest Hematopoietic Progenitor Cell Counts Predict CD34+ Cell Yields in Granulocyte‐Colony‐Stimulating Factor‐Mobilized Peripheral Blood Stem Cell Harvest in Healthy Donors,” Transfusion 50, no. 5 (2010): 1088–1095. - PubMed
-
- Islami M. M., Khan M. A., Aseeri M. A., et al., “Comparison of Biosimilar Filgrastim With Innovator Fligrastim for Peripheral Blood Stem Cells Mobilization, Collection of CD34+ Stem Cells, and Engraftment in Patients Undergoing Autologous and Allogeneic Stem Cell Transplantation: A Single‐Center Experience,” Annals of Transplantation 28 (2023): e938585. - PMC - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

