Human asthenozoospermia: Update on genetic causes, patient management, and clinical strategies
- PMID: 39748639
- PMCID: PMC12183016
- DOI: 10.1111/andr.13828
Human asthenozoospermia: Update on genetic causes, patient management, and clinical strategies
Abstract
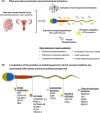
Background: In mammals, sperm fertilization potential relies on efficient progression within the female genital tract to reach and fertilize the oocyte. This fundamental property is supported by the flagellum, an evolutionarily conserved organelle, which contains dynein motor proteins that provide the mechanical force for sperm propulsion and motility. Primary motility of the sperm cells is acquired during their transit through the epididymis and hyperactivated motility is acquired throughout the journey in the female genital tract by a process called capacitation. These activation processes rely on the micro-environment of the genital tracts. In particular, during capacitation, a panoply of ion transporters located at the surface of the sperm cells mediate complex ion exchanges, which induce an increase in plasma membrane fluidity, the alkalinization of the cytoplasm and protein phosphorylation cascades that are compulsory for sperm hyperactivation and fertilization potential. As a consequence, both structural and functional defects of the sperm flagellum can affect sperm motility, resulting in asthenozoospermia, which constitutes the most predominant pathological condition associated with human male infertility.
Objectives: Herein, we have performed a literature review to provide a comprehensive description of the recent advances in the genetics of human asthenozoospermia.
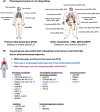
Results and discussion: We describe the currently knowledge on gene mutations that affect sperm morphology and motility, namely, asthenoteratozoospermia; we also specify the gene mutations that exclusively affect sperm function and activation, resulting in functional asthenozoospermia. We discuss the benefit of this knowledge for patient and couple management, in terms of genetic counselling and diagnosis of male infertility as a sole phenotype or in association with ciliary defects. Last, we discuss the current strategies that have been initiated for the development of potential therapeutical and contraceptive strategies targeting genes that are essential for sperm function and activation.
Keywords: asthenozoospermia; contraception; flagellar morphology; gene mutation; signaling; therapeutics.
© 2025 The Author(s). Andrology published by Wiley Periodicals LLC on behalf of American Society of Andrology and European Academy of Andrology.
Conflict of interest statement
The authors declare they have no conflicts of interest.
Figures


References
-
- Yeung CH, Cooper TG. Developmental changes in signalling transduction factors in maturing sperm during epididymal transit. Cell Mol Biol (Noisy‐le‐grand). 2003;49(3):341‐349. - PubMed
-
- Fraser LR. Requirements for successful mammalian sperm capacitation and fertilization. Arch Pathol Lab Med. 1992;116(4):345‐350. - PubMed
-
- Hermo L, Pelletier RM, Cyr DG, Smith CE. Surfing the wave, cycle, life history, and genes/proteins expressed by testicular germ cells. Part 1: background to spermatogenesis, spermatogonia, and spermatocytes. Microsc Res Tech. 2010;73(4):241‐278. - PubMed
-
- Cooper TG, Yeung CH. Acquisition of volume regulatory response of sperm upon maturation in the epididymis and the role of the cytoplasmic droplet. Microsc Res Tech. 2003;61(1):28‐38. - PubMed
-
- Tash JS. Protein phosphorylation: the second messenger signal transducer of flagellar motility. Cell Motil Cytoskeleton. 1989;14(3):332‐339. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

