Prostatic Escherichia coli infection drives CCR2-dependent recruitment of fibrocytes and collagen production
- PMID: 39748675
- PMCID: PMC11789281
- DOI: 10.1242/dmm.052012
Prostatic Escherichia coli infection drives CCR2-dependent recruitment of fibrocytes and collagen production
Abstract
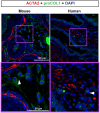
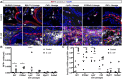
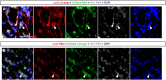
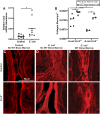
Prostate fibrosis contributes to lower urinary tract dysfunction (LUTD). To develop targeted treatments for prostate fibrosis, it is necessary to identify the cell types and molecular pathways required for collagen production. We used a genetic approach to label and track potential collagen-producing cell lineages in mouse prostate through a round of Escherichia coli UTI89-mediated prostate inflammation. E. coli increased collagen density and production in Gli1+, S100a4+, Lyz2+ and Cd2+ cell lineages, but not in Myh11+ or Srd5a2+ cell lineages, in the mouse prostate. Molecular phenotyping revealed GLI1+LYZ+S100A4+ cells (fibrocytes) in histologically inflamed human prostate. These fibrocytes colocalized with regions of increased collagen in men with LUTD. Fibrocyte recruitment and collagen synthesis was impaired in Ccr2 null mice but restored by allotransplantation of Rosa-GFP donor bone marrow-derived cells. These results suggest that bone marrow-derived fibrocytes are a mediator of prostatic collagen accumulation.
Keywords: CCR2; Fibrocyte; Fibrosis; LUTD; Myofibroblast; Prostate.
© 2025. Published by The Company of Biologists.
Conflict of interest statement
Competing interests The authors declare no competing or financial interests.
Figures



References
-
- Alluri, L. S. C., Paes Batista Da Silva, A., Verma, S., Fu, P., Shen, D. L., MacLennan, G., Gupta, S. and Bissada, N. F. (2021). Presence of specific periodontal pathogens in prostate gland diagnosed with chronic inflammation and adenocarcinoma. Cureus 13, e17742. 10.7759/cureus.17742 - DOI - PMC - PubMed
-
- Andersson-Sjöland, A., De Alba, C. G., Nihlberg, K., Becerril, C., Ramírez, R., Pardo, A., Westergren-Thorsson, G. and Selman, M. (2008). Fibrocytes are a potential source of lung fibroblasts in idiopathic pulmonary fibrosis. Int. J. Biochem. Cell Biol. 40, 2129-2140. 10.1016/j.biocel.2008.02.012 - DOI - PubMed
-
- Bauman, T. M., Nicholson, T. M., Abler, L. L., Eliceiri, K. W., Huang, W., Vezina, C. M. and Ricke, W. A. (2014). Characterization of fibrillar collagens and extracellular matrix of glandular benign prostatic hyperplasia nodules. PLoS ONE 9, e109102. 10.1371/journal.pone.0109102 - DOI - PMC - PubMed
-
- Bell-Cohn, A., Mazur, D. J., Hall, C. C., Schaeffer, A. J. and Thumbikat, P. (2019). Uropathogenic Escherichia coli-induced fibrosis, leading to lower urinary tract symptoms, is associated with type-2 cytokine signaling. Am. J. Physiol. Renal. Physiol. 316, F682-F692. 10.1152/ajprenal.00222.2018 - DOI - PMC - PubMed
MeSH terms
Substances
Grants and funding
- F30DK122686/DK/NIDDK NIH HHS/United States
- R01DK115477/DK/NIDDK NIH HHS/United States
- University of Wisconsin Madison
- F31 ES028594/ES/NIEHS NIH HHS/United States
- U54DK104310/DK/NIDDK NIH HHS/United States
- F31ES028594/ES/NIEHS NIH HHS/United States
- R01CA227542/CA/NCI NIH HHS/United States
- R01 DK115477/DK/NIDDK NIH HHS/United States
- U54 DK104310/DK/NIDDK NIH HHS/United States
- U01DK110807/DK/NIDDK NIH HHS/United States
- U01 DK110807/DK/NIDDK NIH HHS/United States
- R01 ES001332/ES/NIEHS NIH HHS/United States
- T32 OD010957/OD/NIH HHS/United States
- R01 CA227542/CA/NCI NIH HHS/United States
- F30 DK122686/DK/NIDDK NIH HHS/United States
- P30 CA142543/CA/NCI NIH HHS/United States
- R01ES001332/ES/NIEHS NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical
Research Materials

